Vitamin K
| Vitamin K | |
|---|---|
| Drug class | |
| External links | |
| Drugs.com | Medical Encyclopedia |
Vitamin K refers to structurally similar, fat-soluble vitamers found in foods and marketed as dietary supplements.[1] The human body requires vitamin K for post-synthesis modification of certain proteins that are required for blood coagulation (K from koagulation, Danish for "coagulation") or for controlling binding of calcium in bones and other tissues.[2] The complete synthesis involves final modification of these so-called "Gla proteins" by the enzyme gamma-glutamyl carboxylase that uses vitamin K as a cofactor. The presence of uncarboxylated proteins indicates a vitamin K deficiency. Carboxylation allows them to bind (chelate) calcium ions, which they cannot do otherwise.[3] Without vitamin K, blood coagulation is seriously impaired, and uncontrolled bleeding occurs. Research suggests that deficiency of vitamin K may also weaken bones, potentially contributing to osteoporosis, and may promote calcification of arteries and other soft tissues.[2][3][4]
Chemically, the vitamin K family comprises 2-methyl-1,4-naphthoquinone (3-) derivatives. Vitamin K includes two natural vitamers: vitamin K1 (phylloquinone) and vitamin K2 (menaquinone).[3] Vitamin K2, in turn, consists of a number of related chemical subtypes, with differing lengths of carbon side chains made of isoprenoid groups of atoms. The two most studied ones are menaquinone-4 (MK-4) and menaquinone-7 (MK-7).
Vitamin K1 is made by plants, and is found in highest amounts in green leafy vegetables, because it is directly involved in photosynthesis. It is active as a vitamin in animals and performs the classic functions of vitamin K, including its activity in the production of blood-clotting proteins. Animals may also convert it to vitamin K2, variant MK-4. Bacteria in the gut flora can also convert K1 into MK-4. All forms of K2 other than MK-4 can only be produced by bacteria, which use these during anaerobic respiration. Vitamin K3 (menadione), a synthetic form of vitamin K, was used to treat vitamin K deficiency, but because it interferes with the function of glutathione, it is no longer used this way in human nutrition.[2]
Definition
Vitamin K refers to structurally similar, fat-soluble vitamers found in foods and marketed as dietary supplements. "Vitamin K" include several chemical compounds. These are similar in structure in that they share a quinone ring, but differ in the length and degree of saturation of the carbon tail and the number of repeating isoprene units in the side chain (see figures in Chemistry section). Plant-sourced forms are primarily vitamin K1. Animal-sourced foods are primarily vitamin K2.[1][5][6] Vitamin K has several roles: an essential nutrient absorbed from food, a product synthesized and marketed as part of a multi-vitamin or as a single-vitamin dietary supplement, and a prescription medication for specific purposes.[1]
Medical uses
Vitamin deficiency in newborns
Vitamin K is given as an injection to newborns to prevent vitamin K deficiency bleeding.[7] The blood clotting factors of newborn babies are roughly 30–60% that of adult values; this appears to be a consequence of poor transfer of the vitamin across the placenta, and thus low fetal plasma vitamin K.[7] Occurrence of vitamin K deficiency bleeding in the first week of the infant's life is estimated at 0.25–1.7%, with a prevalence of 2–10 cases per 100,000 births. Human milk contains 0.85–9.2 μg/L (median 2.5 μg/L) of vitamin K1, while infant formula is formulated in range of 24–175 μg/L.[7] Late onset bleeding, with onset 2 to 12 weeks after birth, can be a consequence of exclusive breastfeeding, especially if there was no preventive treatment.[7] Late onset prevalence reported at 35 cases per 100,000 live births in infants who had not received prophylaxis at or shortly after birth.[8] Vitamin K deficiency bleeding occurs more frequently in the Asian population compared to the Caucasian population.[7]
Bleeding in infants due to vitamin K deficiency can be severe, leading to hospitalization, brain damage, and death. Intramuscular injection, typically given shortly after birth, is more effective in preventing vitamin K deficiency bleeding than oral administration, which calls for weekly dosing up to three months of age.[7]
Managing warfarin therapy
Warfarin is an anticoagulant drug. It functions by inhibiting an enzyme that is responsible for recycling vitamin K to a functional state. As a consequence, proteins that should be modified by vitamin K are not, including proteins essential to blood clotting, and are thus not functional.[9] The purpose of the drug is to reduce risk of inappropriate blood clotting, which can have serious, potentially fatal consequences.[2] The proper anticoagulant action of warfarin is a function of vitamin K intake and drug dose. Due to differing absorption of the drug and amounts of vitamin K in the diet, dosing must be monitored and individualized for each patient.[10] Some foods are so high in vitamin K1 that medical advice is to avoid those (examples: collard greens, spinach, turnip greens) entirely, and for foods with a modestly high vitamin content, keep consumption as consistent as possible, so that the combination of vitamin intake and warfarin keep the anti-clotting activity in the therapeutic range.[11]
Vitamin K is a treatment for bleeding events caused by overdose of the drug.[12] The vitamin can be administered by mouth, intravenously or subcutaneously.[12] Oral vitamin K is used in situations when a person's International normalised ratio is greater than 10 but there is no active bleeding.[11][13] The newer anticoagulants apixaban, dabigatran and rivaroxaban are not vitamin K antagonists.[14]
Treating rodenticide poisoning
Coumarin is used in the pharmaceutical industry as a precursor reagent in the synthesis of a number of synthetic anticoagulant pharmaceuticals.[15] One subset, 4-hydroxycoumarins, act as vitamin K antagonists. They block the regeneration and recycling of vitamin K. Some of the 4-hydroxycoumarin anticoagulant class of chemicals are designed to have high potency and long residence times in the body, and these are used specifically as second generation rodenticides ("rat poison"). Death occurs after a period of several days to two weeks, usually from internal hemorrhaging.[15] For humans, and for animals that have consumed either the rodenticide or rats poisoned by the rodenticide, treatment is prolonged administration of large amounts of vitamin K.[16][17] This dosing must sometimes be continued for up to nine months in cases of poisoning by "superwarfarin" rodenticides such as brodifacoum. Oral vitamin K1 is preferred over other vitamin K1 routes of administration because it has fewer side effects.[18]
Methods of assessment
An increase in prothrombin time, a coagulation assay, has been used as an indicator of vitamin K status, but it lacks sufficient sensitivity and specificity for this application.[19] Serum phylloquinone is the most commonly used marker of vitamin K status. Concentrations <0.15 µg/L are indicative of deficiency. Disadvantages include exclusion of the other vitamin K vitamers and interference from recent dietary intake.[19] Vitamin K is required for the gamma-carboxylation of specific glutamic acid residues within the Gla domain of the 17 vitamin K–dependent proteins. Thus, a rise in uncarboxylated versions of these proteins is an indirect but sensitive and specific marker for vitamin K deficiency. If uncarboxylated prothrombin is being measured, this "Protein induced by Vitamin K Absence/antagonism (PIVKA-II)" is elevated in vitamin K deficiency. The test is used to assess risk of vitamin K–deficient bleeding in newborn infants.[19] Osteocalcin is involved in calcification of bone tissue. The ratio of uncarboxylated osteocalcin to carboxylated osteocalcin increases with vitamin K deficiency. Vitamin K2 has been shown to lower this ratio and improve lumbar vertebrae bone mineral density.[20] Matrix Gla protein must undergo vitamin K dependent phosphorylation and carboxylation. Elevated plasma concentration of dephosphorylated, uncarboxylated MGP is indicative of vitamin K deficiency.[21]
Dosage
Doses of 2.5 mg to 10 mg can be given by mouth or by injection.[22] For severe bleeding related to warfarn intravenous use is often recommended.[22]
Side effects
No known toxicity is associated with high oral doses of the vitamin K1 or (vitamin K2) forms of vitamin K, so regulatory agencies from US, Japan and European Union concur that no tolerable upper intake levels needs to be set.[4][23][24] However, vitamin K1 has been associated with severe adverse reactions such as bronchospasm and cardiac arrest when given intravenously. The reaction is described as a nonimmune-mediated anaphylactoid reaction, with incidence of 3 per 10,000 treatments. The majority of reactions occurred when polyoxyethylated castor oil was used as the solubilizing agent.[25]
Vitamin deficiency
Vitamin K deficiency is assessed as a vitamin-responsive failure to convert glutamate amino acids to gamma-carboxyglutamate amino acids in prothrombin, the precursor protein to the enzyme thrombin, which has the functional consequence of increasing prothrombin time – a measure of blood clotting activity – and in severe cases can result in bleeding, either internal or external.[2][4] Normal diets are usually not lacking in vitamin K. Thus, primary deficiency is uncommon in healthy children and adults.[3] One exception is infants, who are at an increased risk of deficiency regardless of the vitamin status of the mother during pregnancy and breast feeding because of poor transfer of the vitamin to the placenta and low amounts of the vitamin in breast milk.[7]
Secondary deficiencies can occur in people who consume adequate amounts but have a malabsorption conditions such as cystic fibrosis, or chronic pancreatitis, and in people who have liver damage or disease, for example due to alcoholic liver disease, as the liver is the primary storage organ for the vitamin. Secondary vitamin K deficiency can also occur in people who have a prescription for a vitamin K antagonist drug such as warfarin.[2][3] A drug associated with increased risk of vitamin K deficiency is cefamandole, although the mechanism is unknown.[26]
Dietary recommendations
The US National Academy of Medicine does not distinguish between K1 and K2 – both are counted as vitamin K. When recommendations were last updated in 1998, sufficient information was not available to establish an estimated average requirement or recommended dietary allowance, terms that exist for most vitamins. In instances such as these, the academy defines adequate intakes (AIs) as amounts that appear to be sufficient to maintain good health, with the understanding that at some later date, AIs will be replaced by more exact information. The current AIs for adult women and men ages 19 and older are 90 and 120 μg/day, respectively, for pregnancy is 90 μg/day, and for lactation is 90 μg/day. For infants up to 12 months, the AI is 2.0–2.5 μg/day; for children ages 1–18 years the AI increases with age from 30 to 75 μg/day. As for safety, the academy sets tolerable upper intake levels (known as "upper limits") for vitamins and minerals when evidence is sufficient. Vitamin K has no upper limit, as human data for adverse effects from high doses are not sufficient.[4]
In the European Union, adequate intake is defined the same way as in the US. For women and men over age 18 the adequate intake is set at 70 μg/day, for pregnancy 70 μg/day, and for lactation 70 μg/day. For children ages 1–17 years, adequate intake values increase with age from 12 to 65 μg/day.[27] Japan set adequate intakes for adult women at 65 μg/day and for men at 75 μg/day.[23] The European Union and Japan also reviewed safety and concluded – as had the United States – that there was insufficient evidence to set an upper limit for vitamin K.[23][24]
For US food and dietary supplement labeling purposes, the amount in a serving is expressed as a percentage of daily value. For vitamin K labeling purposes, 100% of the daily value was 80 μg, but on 27 May 2016 it was revised upwards to 120 μg, to bring it into agreement with the highest value for adequate intake.[28][29] Compliance with the updated labeling regulations was required by 1 January 2020 for manufacturers with US$10 million or more in annual food sales, and by 1 January 2021 for manufacturers with lower volume food sales.[30][31] A table of the old and new adult daily values is provided at Reference Daily Intake.
Fortification
According to the Global Fortification Data Exchange, vitamin K deficiency is so rare that no countries require that foods be fortified.[32] The World Health Organization does not have recommendations on vitamin K fortification.[33]
Sources
Vitamin K1 is primarily from plants, especially leafy green vegetables. Small amounts are provided by animal-sourced foods. Vitamin K2 is primarily from animal-sourced foods, with poultry and eggs much better sources than beef, pork or fish.[6] One exception to the latter is nattō, which is made from bacteria-fermented soybeans. It is a rich food source of vitamin K2 variant MK-7, made by the bacteria.[34]
Vitamin K1
| Plant-sourced[6] | Amount K1 (μg / measure) |
|---|---|
| Collard greens boiled, drained, 1⁄2 cup | 530 |
| Spinach boiled, drained, 1⁄2 cup | 445 |
| Turnip greens boiled, drained, 1⁄2 cup | 425 |
| Spinach raw, 1 cup | 145 |
| Brussels sprouts boiled, drained, 1⁄2 cup | 110 |
| Kale raw, 1 cup | 82 |
| Broccoli boiled, drained, 1⁄2 cup | 81 |
| Asparagus boiled, drained, 4 spears | 48 |
| Kiwifruit peeled, sliced, 1⁄2 cup | 36 |
| Chinese cabbage cooked, 1⁄2 cup | 29 |
| Blueberries frozen, 1⁄2 cup | 21 |
| Carrots raw, chopped, 1 cup | 17 |
| Plant-sourced[6] | Amount K1 (μg / measure) |
|---|---|
| Hazelnuts chopped, 1 cup | 16 |
| Grapes, 1⁄2 cup | 11 |
| Tomato products, 1 cup | 9.2 |
| Olive oil, 1.0 tablespoon | 8.1 |
| Zucchini boiled, drained, 1.0 cup | 7.6 |
| Mangos pieces, 1.0 cup | 6.9 |
| Pears, pieces, 1.0 cup | 6.2 |
| Potato baked, including skin, one | 6.0 |
| Sweet potato baked, one | 2.6 |
| Bread whole wheat, 1 slice | 2.5 |
| Bread white, 1 slice | 2.2 |
| Animal-sourced[6] | Amount K1 (μg / measure) |
|---|---|
| Chicken, 4.0 oz | 2.7–3.3 |
| Mollusks, 4 oz | 2.2 |
| Cheese diced, 1⁄2 cup | 1.4–1.7 |
| Beef, 4 oz | 0.9 |
| Pork sausage, 4 oz | 0.9 |
| Yogurt whole milk, 1.0 cup | 0.4 |
| Milk whole or low fat, 1.0 cup | 0.2 |
| Fish, 4 oz | 0.1 |
| Eggs, one | 0.1 |
| Human milk, per liter | 0.85–9.2 (median 2.5)[7] |
Vitamin K2
Animal-sourced foods are a source of vitamin K2[35][36] The MK-4 form is from conversion of plant-sourced vitamin K1 in various tissues in the body.[37]
Chemistry
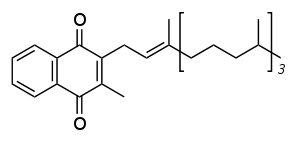
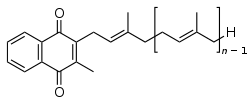
The structure of phylloquinone, Vitamin K1, is marked by the presence of a phytyl sidechain.[4] The structures of menaquinones, vitamin K2, are marked by the polyisoprenyl side chain present in the molecule that can contain four to 13 isoprenyl units. MK-4 is the most common form.[4]
.jpg.webp)
Conversion of vitamin K1 to vitamin K2
In animals, the MK-4 form of vitamin K2 is produced by conversion of vitamin K1 in the testes, pancreas, and arterial walls.[37] While major questions still surround the biochemical pathway for this transformation, the conversion is not dependent on gut bacteria, as it occurs in germ-free rats[38] and in parenterally administered K1 in rats.[39][40] There is evidence that the conversion proceeds by removal of the phytyl tail of K1 to produce menadione (also referred to as vitamin K3) as an intermediate, which is then prenylated to produce MK-4.[41]
Physiology
In animals, vitamin K is involved in the carboxylation of certain glutamate residues in proteins to form gamma-carboxyglutamate (Gla) residues. The modified residues are often (but not always) situated within specific protein domains called Gla domains. Gla residues are usually involved in binding calcium, and are essential for the biological activity of all known Gla proteins.[42]
17 human proteins with Gla domains have been discovered; they play key roles in the regulation of three physiological processes:
- Blood coagulation: prothrombin (factor II), factors VII, IX, and X, and proteins C, S, and Z[43]
- Bone metabolism: osteocalcin, matrix Gla protein (MGP),[44] periostin,[45] and Gla-rich protein.[46][47]
- Vascular biology: Matrix Gla protein, growth arrest – specific protein 6 (Gas6)[48]
- Unknown functions: proline-rich γ-carboxyglutamyl proteins 1 and 2, and transmembrane γ-carboxy glutamyl proteins 3 and 4.[49]
Absorption
Vitamin K is absorbed through the jejunum and ileum in the small intestine. The process requires bile and pancreatic juices. Estimates for absorption are on the order of 80% for vitamin K1 in its free form (as a dietary supplement) but much lower when present in foods. For example, the absorption of vitamin K from kale and spinach – foods identified as having a high vitamin K content – are on the order of 4% to 17% regardless of whether raw or cooked.[3] Less information is available for absorption of vitamin K2 from foods.[3][4]
The intestinal membrane protein Niemann–Pick C1-like 1 (NPC1L1) mediates cholesterol absorption. Animal studies show that it also factors into absorption of vitamins E and K1.[50] The drug ezetimibe inhibits NPC1L1 causing a reduction in cholesterol absorption in humans, and in animal studies, also reduces vitamin E and vitamin K1 absorption. An expected consequence would be that administration of ezetimibe to people who take warfarin (a vitamin K antagonist) would potentiate the warfarin effect. This has been confirmed in humans.[50]
Biochemistry
Function in animals
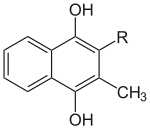

Vitamin K is distributed differently within animals depending on its specific homologue. Vitamin K1 is mainly present in the liver, heart and pancreas, while MK-4 is better represented in the kidneys, brain and pancreas. The liver also contains longer chain homologues MK-7 to MK-13.[51]
The function of vitamin K2 in the animal cell is to add a carboxylic acid functional group to a glutamate (Glu) amino acid residue in a protein, to form a gamma-carboxyglutamate (Gla) residue. This is a somewhat uncommon posttranslational modification of the protein, which is then known as a "Gla protein". The presence of two −COOH (carboxylic acid) groups on the same carbon in the gamma-carboxyglutamate residue allows it to chelate calcium ions. The binding of calcium ions in this way very often triggers the function or binding of Gla-protein enzymes, such as the so-called vitamin K–dependent clotting factors discussed below.[52]
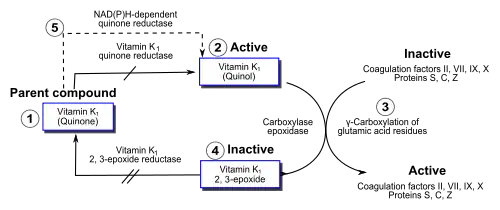
Within the cell, vitamin K participates in a cyclic process. The vitamin undergoes electron reduction to a reduced form called vitamin K hydroquinone, catalyzed by the enzyme vitamin K epoxide reductase (VKOR).[53] Another enzyme then oxidizes vitamin K hydroquinone to allow carboxylation of Glu to Gla; this enzyme is called gamma-glutamyl carboxylase[54] or the vitamin K–dependent carboxylase. The carboxylation reaction only proceeds if the carboxylase enzyme is able to oxidize vitamin K hydroquinone to vitamin K epoxide at the same time. The carboxylation and epoxidation reactions are said to be coupled. Vitamin K epoxide is then restored to vitamin K by VKOR. The reduction and subsequent reoxidation of vitamin K coupled with carboxylation of Glu is called the vitamin K cycle.[55] Humans are rarely deficient in vitamin K because, in part, vitamin K2 is continuously recycled in cells.[56]
Warfarin and other 4-hydroxycoumarins block the action of VKOR.[9] This results in decreased concentrations of vitamin K and vitamin K hydroquinone in tissues, such that the carboxylation reaction catalyzed by the glutamyl carboxylase is inefficient. This results in the production of clotting factors with inadequate Gla. Without Gla on the amino termini of these factors, they no longer bind stably to the blood vessel endothelium and cannot activate clotting to allow formation of a clot during tissue injury. As it is impossible to predict what dose of warfarin will give the desired degree of clotting suppression, warfarin treatment must be carefully monitored to avoid underdose and overdose.[10]
Gamma-carboxyglutamate proteins
The following human Gla-containing proteins ("Gla proteins") have been characterized to the level of primary structure: blood coagulation factors II (prothrombin), VII, IX, and X, anticoagulant protein C and protein S, and the factor X-targeting protein Z. The bone Gla protein osteocalcin, the calcification-inhibiting matrix Gla protein (MGP), the cell growth regulating growth arrest specific gene 6 protein, and the four transmembrane Gla proteins, the function of which is at present unknown. The Gla domain is responsible for high-affinity binding of calcium ions (Ca2+) to Gla proteins, which is often necessary for their conformation, and always necessary for their function.[52]
Gla proteins are known to occur in a wide variety of vertebrates: mammals, birds, reptiles, and fish. The venom of a number of Australian snakes acts by activating the human blood-clotting system. In some cases, activation is accomplished by snake Gla-containing enzymes that bind to the endothelium of human blood vessels and catalyze the conversion of procoagulant clotting factors into activated ones, leading to unwanted and potentially deadly clotting.[57]
Another interesting class of invertebrate Gla-containing proteins is synthesized by the fish-hunting snail Conus geographus.[58] These snails produce a venom containing hundreds of neuroactive peptides, or conotoxins, which is sufficiently toxic to kill an adult human. Several of the conotoxins contain two to five Gla residues.[59]
Function in plants
Vitamin K1 is an important chemical in green plants, where it functions as an electron acceptor in photosystem I during photosynthesis.[60] For this reason, vitamin K1 is found in large quantities in the photosynthetic tissues of plants (green leaves, and dark green leafy vegetables such as romaine lettuce, kale, and spinach), but it occurs in far smaller quantities in other plant tissues.[6][60]
Function in bacteria
Many bacteria, including Escherichia coli found in the large intestine, can synthesize vitamin K2 (MK-7 up to MK-11),[61] but not vitamin K1. Green algae and some species of cyanobacteria (sometimes referred to as blue-green algae) are able to synthesize vitamin K1.[60] In the vitamin K2 synthesizing bacteria, menaquinone transfers two electrons between two different small molecules, during oxygen-independent metabolic energy production processes (anaerobic respiration).[62] For example, a small molecule with an excess of electrons (also called an electron donor) such as lactate, formate, or NADH, with the help of an enzyme, passes two electrons to menaquinone. The menaquinone, with the help of another enzyme, then transfers these two electrons to a suitable oxidant, such as fumarate or nitrate (also called an electron acceptor). Adding two electrons to fumarate or nitrate converts the molecule to succinate or nitrite plus water, respectively.[62] Some of these reactions generate a cellular energy source, ATP, in a manner similar to eukaryotic cell aerobic respiration, except the final electron acceptor is not molecular oxygen, but fumarate or nitrate. In aerobic respiration, the final oxidant is molecular oxygen, which accepts four electrons from an electron donor such as NADH to be converted to water. E. coli, as facultative anaerobes, can carry out both aerobic respiration and menaquinone-mediated anaerobic respiration.[62]
History
In 1929, Danish scientist Henrik Dam investigated the role of cholesterol by feeding chickens a cholesterol-depleted diet.[63] He initially replicated experiments reported by scientists at the Ontario Agricultural College.[64] McFarlane, Graham and Richardson, working on the chick feed program at OAC, had used chloroform to remove all fat from chick chow. They noticed that chicks fed only fat-depleted chow developed hemorrhages and started bleeding from tag sites.[65] Dam found that these defects could not be restored by adding purified cholesterol to the diet. It appeared that – together with the cholesterol – a second compound had been extracted from the food, and this compound was called the coagulation vitamin. The new vitamin received the letter K because the initial discoveries were reported in a German journal, in which it was designated as Koagulationsvitamin. Edward Adelbert Doisy of Saint Louis University did much of the research that led to the discovery of the structure and chemical nature of vitamin K.[66] Dam and Doisy shared the 1943 Nobel Prize for medicine for their work on vitamin K1 and K2 published in 1939. Several laboratories synthesized the compound(s) in 1939.[67]
For several decades, the vitamin K–deficient chick model was the only method of quantifying vitamin K in various foods: the chicks were made vitamin K–deficient and subsequently fed with known amounts of vitamin K–containing food. The extent to which blood coagulation was restored by the diet was taken as a measure for its vitamin K content. Three groups of physicians independently found this: Biochemical Institute, University of Copenhagen (Dam and Johannes Glavind), University of Iowa Department of Pathology (Emory Warner, Kenneth Brinkhous, and Harry Pratt Smith), and the Mayo Clinic (Hugh Butt, Albert Snell, and Arnold Osterberg).[68]
The first published report of successful treatment with vitamin K of life-threatening hemorrhage in a jaundiced patient with prothrombin deficiency was made in 1938 by Smith, Warner, and Brinkhous.[69]
The precise function of vitamin K was not discovered until 1974, when prothrombin, a blood coagulation protein, was confirmed to be vitamin K dependent. When the vitamin is present, prothrombin has amino acids near the amino terminus of the protein as γ-carboxyglutamate instead of glutamate, and is able to bind calcium, part of the clotting process.[70]
Other uses
Forms not found in nature, and thus not "vitamins" are menadione and 4-Amino-2-methyl-1-naphthol ("K5"). Menadione, a synthetic compound sometimes referred to as vitamin K3, is used in the pet food industry because once consumed it is converted to vitamin K2.[71] The US Food and Drug Administration has banned this form from sale as a human dietary supplement because large doses have been shown to cause allergic reactions, hemolytic anemia, and cytotoxicity in liver cells.[2] Research with K5 suggests it may inhibit fungal growth in fruit juices.[72]
Research
Osteoporosis
Vitamin K is required for the gamma-carboxylation of osteocalcin in bone.[73] The risk of osteoporosis, assessed via bone mineral density and fractures, was not affected for people on warfarin therapy – a vitamin K antagonist.[74] Higher dietary intake of vitamin K1 may modestly decrease the risk of fractures.[75] However, there is mixed evidence to support a claim that vitamin K supplementation reduces risk of bone fractures.[3][73][76] For women who were post-menopausal and for all people diagnosed with osteoporosis, supplementation trials reported increases in bone mineral density, a reduction to the odds of any clinical fractures but no significant difference for vertebral fractures.[76] There is a subset of literature on supplementation with vitamin K2 MK-4 and bone health. A meta-analysis reported a decrease in the ratio of uncarboxylated osteocalcin to carboxylated, an increase in lumbar spine bone mineral density, but no significant differences for vertebral fractures.[20]
Cardiovascular health
Matrix Gla protein is a vitamin K-dependent protein found in bone, but also in soft tissues such as arteries, where it appears to function as an anti-calcification protein. In animal studies, animals that lack the gene for MGP exhibit calcification of arteries and other soft tissues.[3] In humans, Keutel syndrome is a rare recessive genetic disorder associated with abnormalities in the gene coding for MGP and characterized by abnormal diffuse cartilage calcification.[77] These observations led to a theory that in humans, inadequately carboxylated MGP, due to low dietary intake of the vitamin, could result in increased risk of arterial calcification and coronary heart disease.[3]
In meta-analyses of population studies, low intake of vitamin K was associated with inactive MGP, arterial calcification[78] and arterial stiffness.[79][80] Lower dietary intakes of vitamin K1 and vitamin K2 were also associated with higher coronary heart disease.[21][81] When blood concentration of circulating vitamin K1 was assessed there was an increased risk in all cause mortality linked to low concentration.[82][83] In contrast to these population studies, a review of randomized trials using supplementation with either vitamin K1 or vitamin K2 reported no role in mitigating vascular calcification or reducing arterial stiffness. The trials were too short to assess any impact on coronary heart disease or mortality.[84]
Other
Population studies suggest that vitamin K status may have roles in inflammation, brain function, endocrine function and an anti-cancer effect. For all of these, there is not sufficient evidence from intervention trials to draw any conclusions.[3] From a review of observational trials, long-term use of vitamin K antagonists as anticoagulation therapy is associated with lower cancer incidence in general.[85] There are conflicting reviews as to whether agonists reduce the risk of prostate cancer.[86][87]
References
- 1 2 3 "Fact Sheet for Health Professionals – Vitamin K". US National Institutes of Health, Office of Dietary Supplements. June 2020. Archived from the original on 29 April 2021. Retrieved 26 August 2020.
- 1 2 3 4 5 6 7 "Vitamin K". Corvallis, OR: Micronutrient Information Center, Linus Pauling Institute, Oregon State University. July 2014. Archived from the original on 7 April 2010. Retrieved 20 March 2017.
- 1 2 3 4 5 6 7 8 9 10 11 BP Marriott; DF Birt; VA Stallings; AA Yates, eds. (2020). "Vitamin K". Present Knowledge in Nutrition, Eleventh Edition. London, United Kingdom: Academic Press (Elsevier). pp. 137–54. ISBN 978-0-323-66162-1.
- 1 2 3 4 5 6 7 Institute of Medicine (US) Panel on Micronutrients (2001). "Vitamin K". Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academy Press. pp. 162–196. doi:10.17226/10026. ISBN 978-0-309-07279-3. PMID 25057538. Archived from the original on 17 April 2021. Retrieved 4 March 2021.
- ↑ "Nutrition facts, calories in food, labels, nutritional information and analysis". Nutritiondata.com. 13 February 2008. Archived from the original on 6 July 2010. Retrieved 21 April 2013.
- 1 2 3 4 5 6 "USDA National Nutrient Database for Standard Reference Legacy: Vitamin K" (PDF). U.S. Department of Agriculture, Agricultural Research Service. 2018. Archived (PDF) from the original on 17 October 2020. Retrieved 27 September 2020.
- 1 2 3 4 5 6 7 8 Mihatsch WA, Braegger C, Bronsky J, Campoy C, Domellöf M, Fewtrell M, Mis NF, Hojsak I, Hulst J, Indrio F, Lapillonne A, Mlgaard C, Embleton N, van Goudoever J (July 2016). "Prevention of Vitamin K Deficiency Bleeding in Newborn Infants: A Position Paper by the ESPGHAN Committee on Nutrition" (PDF). Journal of Pediatric Gastroenterology and Nutrition. 63 (1): 123–9. doi:10.1097/MPG.0000000000001232. PMID 27050049. S2CID 4499477. Archived (PDF) from the original on 13 March 2021. Retrieved 4 March 2021.
- ↑ Sankar MJ, Chandrasekaran A, Kumar P, Thukral A, Agarwal R, Paul VK (May 2016). "Vitamin K prophylaxis for prevention of vitamin K deficiency bleeding: a systematic review". J Perinatol. 36 Suppl 1: S29–35. doi:10.1038/jp.2016.30. PMC 4862383. PMID 27109090.
- 1 2 Whitlon DS, Sadowski JA, Suttie JW (April 1978). "Mechanism of coumarin action: significance of vitamin K epoxide reductase inhibition". Biochemistry. 17 (8): 1371–7. doi:10.1021/bi00601a003. PMID 646989.
- 1 2 Gong IY, Schwarz UI, Crown N, Dresser GK, Lazo-Langner A, Zou G, Roden DM, Stein CM, Rodger M, Wells PS, Kim RB, Tirona RG (November 2011). "Clinical and genetic determinants of warfarin pharmacokinetics and pharmacodynamics during treatment initiation". PLOS ONE. 6 (11): e27808. Bibcode:2011PLoSO...627808G. doi:10.1371/journal.pone.0027808. PMC 3218053. PMID 22114699.
- 1 2 "Important Information to Know When You Are Taking: Warfarin (Coumadin) and Vitamin K" (PDF). National Institute of Health Clinical Center Drug-Nutrient Interaction Task Force. Archived from the original (PDF) on 5 April 2019. Retrieved 17 April 2015.
- 1 2 Tomaselli GF, Mahaffey KW, Cuker A, Dobesh PP, Doherty JU, Eikelboom JW, et al. (December 2017). "2017 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways". Journal of the American College of Cardiology. 70 (24): 3042–3067. doi:10.1016/j.jacc.2017.09.1085. PMID 29203195.
- ↑ Wigle P, Hein B, Bernheisel CR (October 2019). "Anticoagulation: Updated Guidelines for Outpatient Management". Am Fam Physician. 100 (7): 426–34. PMID 31573167.
- ↑ Pengo V, Crippa L, Falanga A, Finazzi G, et al. (November 2011). "Questions and answers on the use of dabigatran and perspectives on the use of other new oral anticoagulants in patients with atrial fibrillation. A consensus document of the Italian Federation of Thrombosis Centers (FCSA)". Thromb. Haemost. 106 (5): 868–76. doi:10.1160/TH11-05-0358. PMID 21946939.
- 1 2 "Coumarin". PubChem, National Library of Medicine, US National Institutes of Health. 4 April 2019. Archived from the original on 5 September 2015. Retrieved 13 April 2019.
- ↑ Bateman DN, Page CB (March 2016). "Antidotes to coumarins, isoniazid, methotrexate and thyroxine, toxins that work via metabolic processes". Br J Clin Pharmacol. 81 (3): 437–45. doi:10.1111/bcp.12736. PMC 4767197. PMID 26255881.
- ↑ Lung D (December 2015). Tarabar A (ed.). "Rodenticide Toxicity Treatment & Management". Medscape. WebMD. Archived from the original on 28 February 2021. Retrieved 4 March 2021.
- ↑ Routt Reigart, J.; Roberts, James (2013). Recognition and Management of Pesticide Poisonings: 6th Edition. http://npic.orst.edu/RMPP/rmpp_ch18.pdf. p. 175.
{{cite book}}: External link in|location= - 1 2 3 Card DJ, Gorska R, Harrington DJ (February 2020). "Laboratory assessment of vitamin K status". J. Clin. Pathol. 73 (2): 70–5. doi:10.1136/jclinpath-2019-205997. PMID 31862867. S2CID 209435449.
- 1 2 Su S, He N, Men P, Song C, Zhai S (June 2019). "The efficacy and safety of menatetrenone in the management of osteoporosis: a systematic review and meta-analysis of randomized controlled trials". Osteoporos Int. 30 (6): 1175–86. doi:10.1007/s00198-019-04853-7. PMID 30734066. S2CID 59616051.
- 1 2 Chen HG, Sheng LT, Zhang YB, Cao AL, Lai YW, Kunutsor SK, Jiang L, Pan A (September 2019). "Association of vitamin K with cardiovascular events and all-cause mortality: a systematic review and meta-analysis". Eur J Nutr. 58 (6): 2191–205. doi:10.1007/s00394-019-01998-3. PMID 31119401. S2CID 162181757. Archived from the original on 27 August 2021. Retrieved 4 March 2021.
- 1 2 "Phytonadione Monograph for Professionals". Drugs.com. Archived from the original on 29 December 2016. Retrieved 23 March 2021.
- 1 2 3 Sasaki S (2008). "Dietary Reference Intakes (DRIs) in Japan". Asia Pac J Clin Nutr. 17 Suppl 2: 420–44. PMID 18460442.
- 1 2 "Tolerable Upper Intake Levels For Vitamins And Minerals" (PDF). European Food Safety Authority. 2006. Archived (PDF) from the original on 16 March 2016. Retrieved 4 March 2021.
- ↑ Britt RB, Brown JN (January 2018). "Characterizing the Severe Reactions of Parenteral Vitamin K1". Clinical and Applied Thrombosis/Hemostasis. 24 (1): 5–12. doi:10.1177/1076029616674825. PMC 6714635. PMID 28301903.
- ↑ Wong RS, Cheng G, Chan NP, Wong WS, NG MH (January 2006). "Use of cefoperazone still needs a caution for bleeding from induced vitamin K deficiency". Am J Hematol. 81 (1): 76. doi:10.1002/ajh.20449. PMID 16369967.
- ↑ "Overview on Dietary Reference Values for the EU population as derived by the EFSA Panel on Dietetic Products, Nutrition and Allergies" (PDF). 2017. Archived (PDF) from the original on 28 August 2017. Retrieved 4 March 2021.
- ↑ "Federal Register May 27, 2016 Food Labeling: Revision of the Nutrition and Supplement Facts Labels. FR page 33982" (PDF). Archived (PDF) from the original on 8 August 2016. Retrieved 4 March 2021.
- ↑ "Daily Value Reference of the Dietary Supplement Label Database (DSLD)". Dietary Supplement Label Database (DSLD). Archived from the original on 7 April 2020. Retrieved 16 May 2020.
- ↑ "Changes to the Nutrition Facts Label". U.S. Food and Drug Administration (FDA). 27 May 2016. Archived from the original on 6 May 2018. Retrieved 16 May 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Industry Resources on the Changes to the Nutrition Facts Label". U.S. Food and Drug Administration (FDA). 21 December 2018. Archived from the original on 25 December 2020. Retrieved 16 May 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Map: Count of Nutrients In Fortification Standards". Global Fortification Data Exchange. Archived from the original on 11 April 2019. Retrieved 3 September 2019.
- ↑ Allen L, de Benoist B, Dary O, Hurrell R, Horton S (2006). "Guidelines on food fortification with micronutrients" (PDF). World Health Organization (WHO). Archived (PDF) from the original on 24 December 2006. Retrieved 3 September 2019.
- 1 2 Tarvainen M, Fabritius M, Yang B (March 2019). "Determination of vitamin K composition of fermented food". Food Chem. 275: 515–22. doi:10.1016/j.foodchem.2018.09.136. PMID 30724228.
- 1 2 3 Schurgers LJ, Vermeer C (November 2000). "Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations". Haemostasis. 30 (6): 298–307. doi:10.1159/000054147. PMID 11356998. S2CID 84592720. Archived from the original on 27 August 2021. Retrieved 4 March 2021.
- 1 2 Elder SJ, Haytowitz DB, Howe J, Peterson JW, Booth SL (January 2006). "Vitamin K contents of meat, dairy, and fast food in the U.S. diet". Journal of Agricultural and Food Chemistry. 54 (2): 463–7. doi:10.1021/jf052400h. PMID 16417305.
- 1 2 Shearer MJ, Newman P (October 2008). "Metabolism and cell biology of vitamin K". Thrombosis and Haemostasis. 100 (4): 530–47. doi:10.1160/TH08-03-0147. PMID 18841274.
- ↑ Davidson RT, Foley AL, Engelke JA, Suttie JW (February 1998). "Conversion of dietary phylloquinone to tissue menaquinone-4 in rats is not dependent on gut bacteria". The Journal of Nutrition. 128 (2): 220–3. doi:10.1093/jn/128.2.220. PMID 9446847.
- ↑ Thijssen HH, Drittij-Reijnders MJ (September 1994). "Vitamin K distribution in rat tissues: dietary phylloquinone is a source of tissue menaquinone-4". The British Journal of Nutrition. 72 (3): 415–25. doi:10.1079/BJN19940043. PMID 7947656.
- ↑ Will BH, Usui Y, Suttie JW (December 1992). "Comparative metabolism and requirement of vitamin K in chicks and rats". The Journal of Nutrition. 122 (12): 2354–60. doi:10.1093/jn/122.12.2354. PMID 1453219.
- ↑ Shearer MJ, Newman P (March 2014). "Recent trends in the metabolism and cell biology of vitamin K with special reference to vitamin K cycling and MK-4 biosynthesis". J Lipid Res. 55 (3): 345–62. doi:10.1194/jlr.R045559. PMC 3934721. PMID 24489112.
- ↑ Furie B, Bouchard BA, Furie BC (March 1999). "Vitamin K–dependent biosynthesis of gamma-carboxyglutamic acid". Blood. 93 (6): 1798–808. doi:10.1182/blood.V93.6.1798.406k22_1798_1808. PMID 10068650.
- ↑ Mann KG (August 1999). "Biochemistry and physiology of blood coagulation". Thrombosis and Haemostasis. 82 (2): 165–74. doi:10.1055/s-0037-1615780. PMID 10605701. S2CID 38487783. Archived from the original on 27 August 2021. Retrieved 4 March 2021.
- ↑ Price PA (1988). "Role of vitamin-K–dependent proteins in bone metabolism". Annual Review of Nutrition. 8: 565–83. doi:10.1146/annurev.nu.08.070188.003025. PMID 3060178.
- ↑ Coutu DL, Wu JH, Monette A, Rivard GE, Blostein MD, Galipeau J (June 2008). "Periostin, a member of a novel family of vitamin K–dependent proteins, is expressed by mesenchymal stromal cells". The Journal of Biological Chemistry. 283 (26): 17991–8001. doi:10.1074/jbc.M708029200. PMID 18450759.
- ↑ Viegas CS, Simes DC, Laizé V, Williamson MK, Price PA, Cancela ML (December 2008). "Gla-rich protein (GRP), a new vitamin K–dependent protein identified from sturgeon cartilage and highly conserved in vertebrates". The Journal of Biological Chemistry. 283 (52): 36655–64. doi:10.1074/jbc.M802761200. PMC 2605998. PMID 18836183.
- ↑ Viegas CS, Cavaco S, Neves PL, Ferreira A, João A, Williamson MK, Price PA, Cancela ML, Simes DC (December 2009). "Gla-rich protein is a novel vitamin K–dependent protein present in serum that accumulates at sites of pathological calcifications". The American Journal of Pathology. 175 (6): 2288–98. doi:10.2353/ajpath.2009.090474. PMC 2789615. PMID 19893032.
- ↑ Hafizi S, Dahlbäck B (December 2006). "Gas6 and protein S. Vitamin K–dependent ligands for the Axl receptor tyrosine kinase subfamily". The FEBS Journal. 273 (23): 5231–44. doi:10.1111/j.1742-4658.2006.05529.x. PMID 17064312. S2CID 13383158. Archived from the original on 27 August 2021. Retrieved 4 March 2021.
- ↑ Kulman JD, Harris JE, Xie L, Davie EW (May 2007). "Proline-rich Gla protein 2 is a cell-surface vitamin K–dependent protein that binds to the transcriptional coactivator Yes-associated protein". Proceedings of the National Academy of Sciences of the United States of America. 104 (21): 8767–72. Bibcode:2007PNAS..104.8767K. doi:10.1073/pnas.0703195104. PMC 1885577. PMID 17502622.
- 1 2 Yamanashi Y, Takada T, Kurauchi R, Tanaka Y, Komine T, Suzuki H (April 2017). "Transporters for the Intestinal Absorption of Cholesterol, Vitamin E, and Vitamin K". J. Atheroscler. Thromb. 24 (4): 347–59. doi:10.5551/jat.RV16007. PMC 5392472. PMID 28100881.
- ↑ Fusaro, M; Mereu, MC; Aghi, A; Iervasi, G; Gallieni, M (May 2017). "Vitamin K and bone". Clinical Cases in Mineral and Bone Metabolism. 14 (2): 200–206. doi:10.11138/ccmbm/2017.14.1.200. PMC 5726210. PMID 29263734.
- 1 2 "Gamma-carboxyglutamic acid-rich (GLA) domain (IPR000294) < InterPro < EMBL-EBI". www.ebi.ac.uk. Archived from the original on 1 April 2021. Retrieved 22 December 2015.
- ↑ Oldenburg J, Bevans CG, Müller CR, Watzka M (2006). "Vitamin K epoxide reductase complex subunit 1 (VKORC1): the key protein of the vitamin K cycle". Antioxidants & Redox Signaling. 8 (3–4): 347–53. doi:10.1089/ars.2006.8.347. PMID 16677080.
- ↑ Presnell SR, Stafford DW (June 2002). "The vitamin K–dependent carboxylase". Thrombosis and Haemostasis. 87 (6): 937–46. doi:10.1055/s-0037-1613115. PMID 12083499.
- ↑ Stafford DW (August 2005). "The vitamin K cycle". Journal of Thrombosis and Haemostasis. 3 (8): 1873–8. doi:10.1111/j.1538-7836.2005.01419.x. PMID 16102054. S2CID 19814205.
- ↑ Rhéaume-Bleue K (2012). Vitamin K2 and the Calcium Paradox. John Wiley & Sons, Canada. p. 79. ISBN 978-1-118-06572-3.
- ↑ Rao VS, Joseph JS, Kini RM (February 2003). "Group D prothrombin activators from snake venom are structural homologues of mammalian blood coagulation factor Xa". Biochem J. 369 (Pt 3): 635–42. doi:10.1042/BJ20020889. PMC 1223123. PMID 12403650.
- ↑ Terlau H, Olivera BM (January 2004). "Conus venoms: a rich source of novel ion channel-targeted peptides". Physiological Reviews. 84 (1): 41–68. doi:10.1152/physrev.00020.2003. PMID 14715910.
- ↑ Buczek O, Bulaj G, Olivera BM (December 2005). "Conotoxins and the posttranslational modification of secreted gene products". Cellular and Molecular Life Sciences. 62 (24): 3067–79. doi:10.1007/s00018-005-5283-0. PMID 16314929. S2CID 25647743.
- 1 2 3 Basset GJ, Latimer S, Fatihi A, Soubeyrand E, Block A (2017). "Phylloquinone (Vitamin K1): Occurrence, Biosynthesis and Functions". Mini Rev Med Chem. 17 (12): 1028–38. doi:10.2174/1389557516666160623082714. PMID 27337968.
- ↑ Bentley R, Meganathan R (September 1982). "Biosynthesis of vitamin K (menaquinone) in bacteria". Microbiological Reviews. 46 (3): 241–80. doi:10.1128/MMBR.46.3.241-280.1982. PMC 281544. PMID 6127606.
- 1 2 3 Haddock BA, Jones CW (March 1977). "Bacterial respiration". Bacteriological Reviews. 41 (1): 47–99. doi:10.1128/mmbr.41.1.47-99.1977. PMC 413996. PMID 140652.
- ↑ Dam CP (1935). "The Antihaemorrhagic Vitamin of the Chick: Occurrence And Chemical Nature". Nature. 135 (3417): 652–653. Bibcode:1935Natur.135..652D. doi:10.1038/135652b0.
- ↑ Dam CP (1941). "The discovery of vitamin K, its biological functions and therapeutical application" (PDF). Nobel Prize Laureate Lecture. Archived (PDF) from the original on 3 March 2016. Retrieved 4 March 2021.
- ↑ McAlister VC (2006). "Control of coagulation: a gift of Canadian agriculture" (PDF). Clinical and Investigative Medicine. 29 (6): 373–377. PMID 17330453. Archived from the original (PDF) on 6 March 2010.
- ↑ MacCorquodale DW, Binkley SB, Thayer SA, Doisy EA (1939). "On the constitution of Vitamin K1". Journal of the American Chemical Society. 61 (7): 1928–1929. doi:10.1021/ja01876a510.
- ↑ Fieser LF (1939). "Synthesis of Vitamin K1". Journal of the American Chemical Society. 61 (12): 3467–3475. doi:10.1021/ja01267a072.
- ↑ Dam CP (12 December 1946). "The discovery of vitamin K, its biological functions and therapeutical application" (PDF). Nobel Prize lecture. Archived (PDF) from the original on 11 December 2011. Retrieved 4 March 2021.
- ↑ Warner ED, Brinkhous KM, Smith HP (1938). "Bleeding Tendency of Obstructive Jaundice". Proceedings of the Society for Experimental Biology and Medicine. 37 (4): 628–630. doi:10.3181/00379727-37-9668P. S2CID 87870462.
- ↑ Stenflo J, Fernlund P, Egan W, Roepstorff P (July 1974). "Vitamin K dependent modifications of glutamic acid residues in prothrombin". Proceedings of the National Academy of Sciences of the United States of America. 71 (7): 2730–3. Bibcode:1974PNAS...71.2730S. doi:10.1073/pnas.71.7.2730. PMC 388542. PMID 4528109.
- ↑ EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) (January 2014). "Scientific Opinion on the safety and efficacy of vitamin K3 (menadione sodium bisulphite and menadione nicotinamide bisulphite) as a feed additive for all animal species". EFSA Journal. 12 (1): 3532. doi:10.2903/j.efsa.2014.3532.
- ↑ Merrifield LS, Yang HY (September 1965). "Vitamin K5 as a fungistatic agent". Appl Microbiol. 13 (5): 660–2. doi:10.1128/AEM.13.5.660-662.1965. PMC 1058320. PMID 5867645.
- 1 2 Hamidi MS, Gajic-Veljanoski O, Cheung AM (2013). "Vitamin K and bone health". Journal of Clinical Densitometry (Review). 16 (4): 409–13. doi:10.1016/j.jocd.2013.08.017. PMID 24090644.
- ↑ Fiordellisi W, White K, Schweizer M (February 2019). "A Systematic Review and Meta-analysis of the Association Between Vitamin K Antagonist Use and Fracture". J Gen Intern Med. 34 (2): 304–11. doi:10.1007/s11606-018-4758-2. PMC 6374254. PMID 30511289.
- ↑ Hao G, Zhang B, Gu M, Chen C, Zhang Q, Zhang G, Cao X (April 2017). "Vitamin K intake and the risk of fractures: A meta-analysis". Medicine (Baltimore). 96 (17): e6725. doi:10.1097/MD.0000000000006725. PMC 5413254. PMID 28445289.
- 1 2 Mott A, Bradley T, Wright K, Cockayne ES, Shearer MJ, Adamson J, Lanham-New SA, Torgerson DJ (August 2019). "Effect of vitamin K on bone mineral density and fractures in adults: an updated systematic review and meta-analysis of randomised controlled trials". Osteoporos Int. 30 (8): 1543–59. doi:10.1007/s00198-019-04949-0. PMID 31076817. S2CID 149445288. Archived from the original on 17 April 2021. Retrieved 4 March 2021.
- ↑ Munroe PB, Olgunturk RO, Fryns JP, et al. (1999). "Mutations in the gene encoding the human matrix Gla protein cause Keutel syndrome". Nat. Genet. 21 (1): 142–4. doi:10.1038/5102. PMID 9916809. S2CID 1244954.
- ↑ Geleijnse JM, Vermeer C, Grobbee DE, Schurgers LJ, Knapen MH, van der Meer IM, Hofman A, Witteman JC (November 2004). "Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study". The Journal of Nutrition. 134 (11): 3100–5. doi:10.1093/jn/134.11.3100. PMID 15514282.
- ↑ Roumeliotis S, Dounousi E, Eleftheriadis T, Liakopoulos V (February 2019). "Association of the Inactive Circulating Matrix Gla Protein with Vitamin K Intake, Calcification, Mortality, and Cardiovascular Disease: A Review". Int J Mol Sci. 20 (3): 628. doi:10.3390/ijms20030628. PMC 6387246. PMID 30717170.
- ↑ Maresz K (February 2015). "Proper Calcium Use: Vitamin K2 as a Promoter of Bone and Cardiovascular Health". Integrative Medicine. 14 (1): 34–39. PMC 4566462. PMID 26770129.
- ↑ Gast GC, de Roos NM, Sluijs I, Bots ML, Beulens JW, Geleijnse JM, Witteman JC, Grobbee DE, Peeters PH, van der Schouw YT (September 2009). "A high menaquinone intake reduces the incidence of coronary heart disease". Nutrition, Metabolism, and Cardiovascular Diseases. 19 (7): 504–10. doi:10.1016/j.numecd.2008.10.004. PMID 19179058.
- ↑ Zhang S, Guo L, Bu C (March 2019). "Vitamin K status and cardiovascular events or mortality: A meta-analysis". Eur J Prev Cardiol. 26 (5): 549–53. doi:10.1177/2047487318808066. PMID 30348006. S2CID 53037302.
- ↑ Shea MK, Barger K, Booth SL, Matuszek G, Cushman M, Benjamin EJ, Kritchevsky SB, Weiner DE (June 2020). "Vitamin K status, cardiovascular disease, and all-cause mortality: a participant-level meta-analysis of 3 US cohorts". Am J Clin Nutr. 111 (6): 1170–77. doi:10.1093/ajcn/nqaa082. PMC 7266692. PMID 32359159.
{{cite journal}}: CS1 maint: PMC embargo expired (link) - ↑ Vlasschaert C, Goss CJ, Pilkey NG, McKeown S, Holden RM (September 2020). "Vitamin K Supplementation for the Prevention of Cardiovascular Disease: Where Is the Evidence? A Systematic Review of Controlled Trials". Nutrients. 12 (10): 2909. doi:10.3390/nu12102909. PMC 7598164. PMID 32977548.
- ↑ Shurrab M, Quinn KL, Kitchlu A, Jackevicius CA, Ko DT (September 2019). "Long-Term Vitamin K Antagonists and Cancer Risk: A Systematic Review and Meta-Analysis". Am. J. Clin. Oncol. 42 (9): 717–24. doi:10.1097/COC.0000000000000571. PMID 31313676. S2CID 197421591.
- ↑ Luo JD, Luo J, Lai C, Chen J, Meng HZ (December 2018). "Is use of vitamin K antagonists associated with the risk of prostate cancer?: A meta-analysis". Medicine (Baltimore). 97 (49): e13489. doi:10.1097/MD.0000000000013489. PMC 6310569. PMID 30544443.
- ↑ Kristensen KB, Jensen PH, Skriver C, Friis S, Pottegård A (April 2019). "Use of vitamin K antagonists and risk of prostate cancer: Meta-analysis and nationwide case-control study" (PDF). Int. J. Cancer. 144 (7): 1522–1529. doi:10.1002/ijc.31886. PMID 30246248. S2CID 52339455. Archived (PDF) from the original on 17 April 2021. Retrieved 4 March 2021.
Further reading
- "Vitamin K: Another Reason to Eat Your Greens" (PDF). Agricultural Research. 48 (1). January 2000. ISSN 2169-8244. Archived (PDF) from the original on 17 April 2021. Retrieved 4 March 2021.
External links
| Look up vitamin k in Wiktionary, the free dictionary. |
- "Vitamin K". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 18 April 2021. Retrieved 4 March 2021.
- "Phylloquinone". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 17 April 2021. Retrieved 4 March 2021.
- "Phytomenadione". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 17 April 2021. Retrieved 4 March 2021.
- "Vitamin K2". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 18 April 2021. Retrieved 4 March 2021.
- "Menadione". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 20 January 2021. Retrieved 4 March 2021.