Tacalcitol
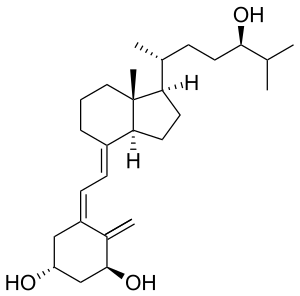 | |
| Names | |
|---|---|
| Trade names | Curatoderm, Bonalfa, others |
| Other names | (1α,24R)-1,24-Dihydroxyvitamin D3 |
IUPAC name
| |
| Clinical data | |
| Drug class | Vitamin D3 analog[1] |
| Main uses | Plaque psoriasis[2] |
| Side effects | Skin rash, high calcium[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | Applied to the skin[2] |
| External links | |
| AHFS/Drugs.com | International Drug Names |
| Chemical and physical data | |
| Formula | C27H44O3 |
| Molar mass | 416.64 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Tacalcitol sold under the brand names Curatoderm among others, is a medication used to treat psoriasis, specifically plaque psoriasis.[2][3] It is applied to the skin once to twice per day.[2][1]
Side effects may include skin rash and high calcium.[2] It is a manufactured vitamin D3 analog.[1]
Tacalcitol was approved for medical use in Japan in 1993.[4] It is on the World Health Organization's List of Essential Medicines as an alternative to calcipotriol.[5] In the United Kingdom 30 grams costs the NHS about £13 as of 2023.[6]
Medical uses
It is usually used to treat psoriasis, chronic chapped lips and other severe dry skin conditions because of its ability to reduce excessive skin cell turnover.[1] It is available as an ointment or lotion.
It has also been used for vitiligo[7][8] and Hailey-Hailey disease.[9]
Mechanism
Tacalcitol reduces excessive cell turnover in the epidermis by interacting with vitamin D receptors on keratinocytes.[10][11]
References
- 1 2 3 4 Peters DC, Balfour JA (August 1997). "Tacalcitol". Drugs. 54 (2): 265–71, discussion 272. doi:10.2165/00003495-199754020-00005. PMID 9257082.
- 1 2 3 4 5 6 "Tacalcitol". NICE. Archived from the original on 10 September 2023. Retrieved 9 September 2023.
- ↑ "eEML - Electronic Essential Medicines List". list.essentialmeds.org. Archived from the original on 10 June 2023. Retrieved 9 September 2023.
- ↑ Tarutani, M (October 2004). "[Vitamin D3 for external application--history of development and clinical application]". Clinical calcium. 14 (10): 124–8. PMID 15577144.
- ↑ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ↑ "Tacalcitol Medicinal forms". NICE. Archived from the original on 10 September 2023. Retrieved 9 September 2023.
- ↑ Leone G, Pacifico A, Iacovelli P, Paro Vidolin A, Picardo M (March 2006). "Tacalcitol and narrow-band phototherapy in patients with vitiligo". Clin. Exp. Dermatol. 31 (2): 200–5. doi:10.1111/j.1365-2230.2005.02037.x. PMID 16487090. S2CID 39021489.
- ↑ Birlea SA, Costin GE, Norris DA (April 2008). "Cellular and molecular mechanisms involved in the action of vitamin D analogs targeting vitiligo depigmentation". Current Drug Targets. 9 (4): 345–59. doi:10.2174/138945008783954970. PMID 18393827.
- ↑ Aoki T, Hashimoto H, Koseki S, Hozumi Y, Kondo S (November 1998). "1alpha,24-dihydroxyvitamin D3 (tacalcitol) is effective against Hailey-Hailey disease both in vivo and in vitro". Br. J. Dermatol. 139 (5): 897–901. doi:10.1046/j.1365-2133.1998.02522.x. PMID 9892963. S2CID 72418207.
- ↑ Matsumoto K, Hashimoto K, Kiyoki M, Yamamoto M, Yoshikawa K (February 1990). "Effect of 1,24R-dihydroxyvitamin D3 on the growth of human keratinocytes". The Journal of Dermatology. 17 (2): 97–103. doi:10.1111/j.1346-8138.1990.tb03714.x. PMID 2158504. S2CID 38248260.
- ↑ Fukuoka M, Sakurai K, Ohta T, Kiyoki M, Katayama I (2001). "Tacalcitol, an active vitamin D3, induces nerve growth factor production in human epidermal keratinocytes". Skin Pharmacol. Appl. Skin Physiol. 14 (4): 226–33. doi:10.1159/000056351. PMID 11464105. S2CID 24302198. Archived from the original on 2012-03-06. Retrieved 2023-05-30.
External links
| Identifiers: |
|
|---|