Hydroquinone
Template:Chembox EUClass
 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Benzene-1,4-diol[1] | |
| Other names | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
Beilstein Reference |
605970 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
Gmelin Reference |
2742 |
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 3077, 2662 |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C6H6O2 |
| Molar mass | 110.112 g·mol−1 |
| Appearance | white solid |
| Density | 1.3 g cm−3, solid |
| Melting point | 172 °C (342 °F; 445 K) |
| Boiling point | 287 °C (549 °F; 560 K) |
Solubility in water |
5.9 g/100 mL (15 °C) |
| Vapor pressure | 10−5 mmHg (20 °C)[2] |
| Acidity (pKa) | 9.9[3] |
Magnetic susceptibility (χ) |
−64.63×10−6 cm3/mol |
| Structure | |
Dipole moment |
1.4±0.1 D[4] |
| Pharmacology | |
| D11AX11 (WHO) | |
| Hazards | |
| R-phrases (outdated) | Template:R22 Template:R40 R41 Template:R43 Template:R50 Template:R68 |
| S-phrases (outdated) | Template:S2 S26 Template:S36/37/39 Template:S61 |
| NFPA 704 (fire diamond) | |
| Flash point | 165 °C (329 °F; 438 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
490 mg/kg (mammal, oral) 245 mg/kg (mouse, oral) 200 mg/kg (rabbit, oral) 320 mg/kg (rat, oral) 550 mg/kg (guinea pig, oral) 200 mg/kg (dog, oral) 70 mg/kg (cat, oral)[6] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 2 mg/m3[2] |
REL (Recommended) |
C 2 mg/m3 [15-minute][2] |
IDLH (Immediate danger) |
50 mg/m3[2] |
| Related compounds | |
Related benzenediols |
Pyrocatechol Resorcinol |
Related compounds |
1,4-benzoquinone |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
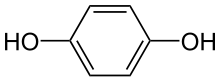
Hydroquinone, also known as benzene-1,4-diol or quinol, is an aromatic organic compound that is a type of phenol, a derivative of benzene, having the chemical formula C6H4(OH)2. It has two hydroxyl groups bonded to a benzene ring in a para position. It is a white granular solid. Substituted derivatives of this parent compound are also referred to as hydroquinones. The name "hydroquinone" was coined by Friedrich Wöhler in 1843.[7]
Production
Hydroquinone is produced industrially in two main ways.[8]
- The most widely used route is similar to the cumene process in reaction mechanism and involves the dialkylation of benzene with propene to give 1,4-diisopropylbenzene. This compound reacts with air to afford the bis(hydroperoxide), which is structurally similar to cumene hydroperoxide and rearranges in acid to give acetone and hydroquinone.[9]
- A second route involves hydroxylation of phenol over a catalyst. The conversion uses hydrogen peroxide and affords a mixture of hydroquinone and catechol (benzene-1,2-diol):
- C6H5OH + H2O2 → C6H4(OH)2 + H2O
Other, less common methods include:
- A potentially significant synthesis of hydroquinone from acetylene and iron pentacarbonyl has been proposed[10][11][12][13][14][15] Iron pentacarbonyl serves as a catalyst, rather than as a reagent, in the presence of free carbon monoxide gas. Rhodium or ruthenium can substitute for iron as the catalyst with favorable chemical yields but are not typically used due to their cost of recovery from the reaction mixture.[10]
- Hydroquinone and its derivatives can also be prepared by oxidation of various phenols. Examples include Elbs persulfate oxidation and Dakin oxidation:
- Hydroquinone was first obtained in 1820 by the French chemists Pelletier and Caventou via the dry distillation of quinic acid.[16]
Reactions
The reactivity of hydroquinone's hydroxyl groups resembles that of other phenols, being weakly acidic. The resulting conjugate base undergoes easy O-alkylation to give mono- and diethers. Similarly, hydroquinone is highly susceptible to ring substitution by Friedel–Crafts reactions such as alkylation. This reaction is exploited en route to popular antioxidants such as 2-tert-butyl-4-methoxyphenol (BHA). The useful dye quinizarin is produced by diacylation of hydroquinone with phthalic anhydride.[8]
Redox
Hydroquinone undergoes oxidation under mild conditions to give benzoquinone. This process can be reversed. Some naturally occurring hydroquinone derivatives exhibit this sort of reactivity, one example being coenzyme Q. Industrially this reaction is exploited both with hydroquinone itself but more often with its derivatives where one OH has been replaced by an amine.
When colorless hydroquinone and benzoquinone, a bright yellow solid, are cocrystallized in a 1:1 ratio, a dark-green crystalline charge-transfer complex (melting point 171 °C) called quinhydrone (C6H6O2·C6H4O2) is formed. This complex dissolves in hot water, where the two molecules dissociate in solution.[17]
Amination
An important reaction is the conversion of hydroquinone to the mono- and diamine derivatives. Methylaminophenol, used in photography, is produced in this way:[8]
- C6H4(OH)2 + CH3NH2 → HOC6H4NHCH3 + H2O
Similarly diamines, useful in the rubber industry as antiozone agents, are produced similarly from aniline:
- C6H4(OH)2 + 2 C6H5NH2 → C6H4(N(H)C6H5)2 + 2 H2O
Uses
Hydroquinone has a variety of uses principally associated with its action as a reducing agent that is soluble in water. It is a major component in most black and white photographic developers for film and paper where, with the compound metol, it reduces silver halides to elemental silver.
There are various other uses associated with its reducing power. As a polymerisation inhibitor, exploiting its antioxidant properties, hydroquinone prevents polymerization of acrylic acid, methyl methacrylate, cyanoacrylate, and other monomers that are susceptible to radical-initiated polymerization. By acting as a free radical scavenger, hydroquinone serves to prolong the shelflife of light-sensitive resins such as preceramic polymers.[18]
Hydroquinone can lose a hydrogen cation from both hydroxyl groups to form a diphenolate ion. The disodium diphenolate salt of hydroquinone is used as an alternating comonomer unit in the production of the polymer PEEK.
Skin depigmentation
Hydroquinone is used as a topical application in skin whitening to reduce the color of skin. It does not have the same predisposition to cause dermatitis as metol does. This is a prescription-only ingredient in some countries, including the member states of the European Union under Directives 76/768/EEC:1976.[19][20]
In 2006, the United States Food and Drug Administration revoked its previous approval of hydroquinone and proposed a ban on all over-the-counter preparations.[21] The FDA stated that hydroquinone cannot be ruled out as a potential carcinogen.[22] This conclusion was reached based on the extent of absorption in humans and the incidence of neoplasms in rats in several studies where adult rats were found to have increased rates of tumours, including thyroid follicular cell hyperplasias, anisokaryosis (variation in nuclei sizes), mononuclear cell leukemia, hepatocellular adenomas and renal tubule cell adenomas. The Campaign for Safe Cosmetics has also highlighted concerns.[23]
Numerous studies have revealed that hydroquinone, if taken orally, can cause exogenous ochronosis, a disfiguring disease in which blue-black pigments are deposited onto the skin; however, skin preparations containing the ingredient are administered topically. The FDA had classified hydroquinone in 1982 as a safe product - generally recognized as safe and effective (GRASE), however additional studies under the National Toxicology Program (NTP) were suggested in order to determine whether there is a risk to humans from the use of hydroquinone.[21][24][22] NTP evaluation showed some evidence of long-term carcinogenic and genotoxic effects[25]
While using hydroquinone as a lightening agent can be effective with proper use, it can also cause skin sensitivity. Using a daily sunscreen with a high PPD (persistent pigment darkening) rating reduces the risk of further damage. Hydroquinone is sometimes combined with alpha-hydroxy acids that exfoliate the skin to quicken the lightening process. In the United States, topical treatments usually contain up to 2% in hydroquinone. Otherwise, higher concentrations (up to 4%) should be prescribed and used with caution.
While hydroquinone remains widely prescribed for treatment of hyperpigmentation, questions raised about its safety profile by regulatory agencies in the EU, Japan, and USA encourage the search for other agents with comparable efficacy.[26] Several such agents are already available or under research,[27] including azelaic acid,[28] kojic acid, retinoids, cysteamine,[29] topical steroids, glycolic acid, and other substances. One of these, 4-butylresorcinol, has been proven to be more effective at treating melanin-related skin disorders by a wide margin, as well as safe enough to be made available over the counter.[30]
Natural occurrences
Hydroquinones are one of the two primary reagents in the defensive glands of bombardier beetles, along with hydrogen peroxide (and perhaps other compounds, depending on the species), which collect in a reservoir. The reservoir opens through a muscle-controlled valve onto a thick-walled reaction chamber. This chamber is lined with cells that secrete catalases and peroxidases. When the contents of the reservoir are forced into the reaction chamber, the catalases and peroxidases rapidly break down the hydrogen peroxide and catalyze the oxidation of the hydroquinones into p-quinones. These reactions release free oxygen and generate enough heat to bring the mixture to the boiling point and vaporize about a fifth of it, producing a hot spray from the beetle's abdomen.[31]
Farnesyl hydroquinone derivatives are the principal irritants exuded by the poodle-dog bush, which can cause severe contact dermatitis in humans.
Hydroquinone is thought to be the active toxin in Agaricus hondensis mushrooms.[32]
Hydroquinone has been shown to be one of the chemical constituents of the natural product propolis.[33]
It is also one of the chemical compounds found in castoreum. This compound is gathered from the beaver's castor sacs.[34]
In bearberry (Arctostaphylos uva-ursi), arbutin is converted to hydroquinone.
See also
- Photographic developer
- Semiquinone
- Bombardier beetle
References
- 1 2 "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 691. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- 1 2 3 4 NIOSH Pocket Guide to Chemical Hazards. "#0338". National Institute for Occupational Safety and Health (NIOSH).
- ↑ "Hydroquinone" (PDF). OECD SIDS. UNEP Publications. Archived from the original (PDF) on 2016-10-20. Retrieved 2021-10-11.
- ↑ Lander, John J.; Svirbely, John J. Lander, W. J. (1945). "The Dipole Moments of Catechol, Resorcinol and Hydroquinone". Journal of the American Chemical Society. 67 (2): 322–324. doi:10.1021/ja01218a051.
- ↑ "Archived copy" (PDF). Archived (PDF) from the original on 2014-02-02. Retrieved 2014-01-25.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ "Hydroquinone". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ↑ F. Wöhler (1844) "Untersuchungen über das Chinon" (Investigations of quinone), Annalen der Chemie und Pharmacie, 51 : 145-163. From page 146: Archived 2019-12-16 at the Wayback Machine "Das so erhaltene Destillat … enthält … einen neuen, krystallisierenden Körper, den ich unter dem Namen farbloses Hydrochinon weiter unten näher beschreiben werde." (The distillate so obtained … contains … a new, crystallizable substance, that I will describe, under the name of colorless hydroquinone, further below in more detail.) [Note: Wöhler's empirical formula for hydroquinone (p. 152) is incorrect because (1) he attributed 25 (instead of 24) carbon atoms to the molecule, and (2) as many chemists at the time did, he used the wrong atomic masses for carbon (6 instead of 12) and oxygen (8 instead of 16). With these corrections, his empirical formula becomes: C12H12O4. Dividing the subscripts by 2, the result is: C6H6O2, which is correct.]
- 1 2 3 Phillip M. Hudnall "Hydroquinone" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. 2005 Wiley-VCH, Weinheim. doi:10.1002/14356007.a13_499.
- ↑ Gerhard Franz, Roger A. Sheldon "Oxidation" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2000 doi:10.1002/14356007.a18_261
- 1 2 Reppe, Walter; Kutepow, N; Magin, A (1969). "Cyclization of Acetylenic Compounds". Angewandte Chemie International Edition in English. 8 (10): 727–733. doi:10.1002/anie.196907271.
- ↑ Hubel, Karl; Braye, Henri (1960). Process for the preparation of substituted cyclic compounds and products resulting therefrom US3149138 A (PDF). Union Carbide Corp. Archived (PDF) from the original on 2016-04-10. Retrieved 2022-03-14.
- ↑ Pino, Piero; Braca, Giuseppe; Sbrana, Glauco (1964). Preparation of hydroquinone US3355503 A (PDF). Lonza Ag. Archived (PDF) from the original on 2016-04-18. Retrieved 2022-03-14.
- ↑ Walter, Reppe; Magin, August (1966). Production of hydroquinones US3394193 A (PDF). Basf Ag. Archived (PDF) from the original on 2016-04-11. Retrieved 2022-03-14.
- ↑ Piero, Pino; Giuseppe, Braca; Frediano, Settimo; Glauco, Sbrana (1967). Preparation of hydroquinone US3459812 A (PDF). Lonza Ag. Archived (PDF) from the original on 2016-04-09. Retrieved 2022-03-14.
- ↑ Holmes, J.; Hagemeyer, H. (1971). Process for the production of hydroquinone US 3742071 A (PDF). Eastman Kodak Co. Archived (PDF) from the original on 2016-04-08. Retrieved 2022-03-14.
- ↑ See:
- Pelletier and Caventou (1820) "Recherches chimiques sur les quinquinas" (Chemical investigations of quinquinas [i.e., the bark of various Cinchona trees]), Annales de Chimie et de Physique, 2nd series, 15 : 289-318, 337-364. On pages 341-342, the preparation and properties of l'acide pyro-kinique (pyroquinic acid or hydroquinone) are discussed.
- Roscoe, Henry (1891). A Treatise on Chemistry, Volume 3, Part 3. London: Macmillan & Co. p. 165. Archived from the original on 2021-04-29. Retrieved 2021-10-11.
- ↑ 1927-, Streitwieser, Andrew (1992). Introduction to organic chemistry. Heathcock, Clayton H., 1936-, Kosower, Edward M. (4th ed.). Upper Saddle River, N.J.: Prentice Hall. ISBN 978-0139738500. OCLC 52836313.
{{cite book}}: CS1 maint: numeric names: authors list (link) - ↑ Additive manufacturing of ceramics from preceramic polymers Archived 2020-08-07 at the Wayback Machine Additive manufacturing 2019 vol. 27. pp 80-90
- ↑ 76/768/EEC:1976 Council Directive 76/768/EEC of 27 July 1976 on the approximation of the laws of the Member States relating to cosmetic products : http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:31976L0768:EN:HTML Archived 2010-11-15 at the Wayback Machine
- ↑ "Clear N Smooth Skin Toning Cream recalled". 2011-10-04. Archived from the original on 2019-01-21. Retrieved 4 April 2018.
- 1 2 United States Food and Drug Administration (2006). Skin Bleaching Drug Products for Over-the-Counter Product Use; Proposed Rule (PDF) (Report). 1978N-0065. Archived (PDF) from the original on 2011-05-16.
- 1 2 Research, Center for Drug Evaluation and. "About the Center for Drug Evaluation and Research - Hydroquinone Studies Under The National Toxicology Program (NTP)". www.fda.gov. Archived from the original on 2017-01-22. Retrieved 2017-02-12.
- ↑ Campaign For Safe Cosmetics - Hydroquinone Archived 2010-11-27 at the Wayback Machine
- ↑ Olumide, YM; Akinkugbe, AO; Altraide, D; Mohammed, T; Ahamefule, N; Ayanlowo, S; Onyekonwu, C; Essen, N (April 2008). "Complications of chronic use of skin lightening cosmetics". International Journal of Dermatology. 47 (4): 344–53. doi:10.1111/j.1365-4632.2008.02719.x. PMID 18377596.
- ↑ "Hydroquinone 10022-H". ntp.niehs.nih.gov. Archived from the original on 2017-10-01. Retrieved 2017-02-12.
- ↑ Draelos, Zoe Diana (2007-09-01). "Skin lightening preparations and the hydroquinone controversy". Dermatologic Therapy. 20 (5): 308–313. doi:10.1111/j.1529-8019.2007.00144.x. ISSN 1529-8019. PMID 18045355. S2CID 24913995.
- ↑ Bandyopadhyay, Debabrata (2009-01-01). "Topical treatment of melasma". Indian Journal of Dermatology. 54 (4): 303–309. doi:10.4103/0019-5154.57602. ISSN 0019-5154. PMC 2807702. PMID 20101327.
- ↑ Mazurek, Klaudia; Pierzchała, Ewa (2016-09-01). "Comparison of efficacy of products containing azelaic acid in melasma treatment". Journal of Cosmetic Dermatology. 15 (3): 269–282. doi:10.1111/jocd.12217. ISSN 1473-2165. PMID 27028014.
- ↑ Mansouri, P.; Farshi, S.; Hashemi, Z.; Kasraee, B. (2015-07-01). "Evaluation of the efficacy of cysteamine 5% cream in the treatment of epidermal melasma: a randomized double-blind placebo-controlled trial". The British Journal of Dermatology. 173 (1): 209–217. doi:10.1111/bjd.13424. ISSN 1365-2133. PMID 25251767. S2CID 21618233.
- ↑ "Hydroquinones". Phenols—Advances in Research and Application: 2013 Edition. Scholastic. 2013. p. 76.
- ↑ Organic Chemistry, Solomon and Fryhle, 10th edition, Wiley Publishing, 2010.
- ↑ Joval, E; Kroeger, P; N (April 1996). "Hydroquinone: the toxic compound of Agaricus hondensis". Planta Medica. 62 (2): 185. doi:10.1055/s-2006-957852. PMID 17252436.
- ↑ Burdock, G.A. (1998). "Review of the biological properties and toxicity of bee propolis (propolis)". Food and Chemical Toxicology. 36 (4): 347–363. doi:10.1016/S0278-6915(97)00145-2. PMID 9651052.
- ↑ The Beaver: Its Life and Impact. Dietland Muller-Schwarze, 2003, page 43 (book at google books Archived 2020-10-09 at the Wayback Machine)
External links
- International Chemical Safety Card 0166 Archived 2021-04-29 at the Wayback Machine
- NIOSH Pocket Guide to Chemical Hazards Archived 2021-09-21 at the Wayback Machine
- IARC Monograph: "Hydroquinone"
