Riboflavin
 | |
 Chemical structure | |
| Names | |
|---|---|
| Trade names | many |
| Other names | vactochrome, lactoflavin, vitamin G[1] |
IUPAC name
| |
| Clinical data | |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | by mouth, IM, IV |
| Defined daily dose | not established[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Elimination half-life | 66 to 84 minutes |
| Excretion | Urine |
| Chemical and physical data | |
| Formula | C17H20N4O6 |
| Molar mass | 376.369 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Riboflavin, also known as vitamin B2, is a vitamin found in food and used as a dietary supplement.[2][4] Food sources include eggs, green vegetables, milk and other dairy product, meat, mushrooms, and almonds.[4] Some countries require its addition to grains.[4][5] As a supplement it is used to prevent and treat riboflavin deficiency and prevent migraines.[2][4] It may be given by mouth or injection.[2]
It is nearly always well tolerated.[2] Normal doses are safe during pregnancy.[2] Riboflavin is in the vitamin B group.[2] It is required by the body for cellular respiration.[2]
Riboflavin was discovered in 1920, isolated in 1933, and first made in 1935.[6] It is on the World Health Organization's List of Essential Medicines.[7] Riboflavin is available as a generic medication and over the counter.[8] In the United States a month of supplements was priced (in 2015) at less than 25 USD.[8]
Definition
Riboflavin, also known as vitamin B2, is a vitamin found in food, sold as a dietary supplement, and used in food fortification programs in countries where deficiency is common.[4][9][10][11][12]
Medical uses

Corneal ectasia is a progressive thinning of the cornea; the most common form of this condition is keratoconus. Collagen cross-linking by applying riboflavin topically then shining UV light is a method to slow progression of corneal ectasia by strengthening corneal tissue.[13]
A 2017 review found that riboflavin taken daily in amounts roughly 200 to 400 times the Recommended Dietary Allowance (RDA) may be useful to prevent migraines in adults, but found that clinical trials in adolescents and children had mixed outcomes.[14] A hypothesis has been proposed that riboflavin improves mitochondrial energy production.[15]
Dosage
The defined daily dose is not established.[3]
Pharmacokinetics
The body absorbs little riboflavin from single doses beyond 27 mg.[4][16] When excess amounts are consumed, they are either not absorbed or the small amount that is absorbed is excreted in urine.[9]
After a single oral dose, biologic half-life is about 66 to 84 minutes in healthy people.[16]
Side effects
In humans, there is no evidence for riboflavin toxicity produced by excessive intakes, in part because it has lower water solubility than other B vitamins, because absorption becomes less efficient as doses increase, and because what exceeds the absorption is excreted via the kidneys into urine.[17] Even when 400 mg of riboflavin per day was given orally to subjects in one study for three months to investigate the efficacy of riboflavin in the prevention of migraine headache, no short-term side effects were reported.[18] Any excess at nutritionally relevant doses is excreted in the urine,[19] imparting a bright yellow color when in large quantities. The limited data available on riboflavin's adverse effects do not mean, however, that high intakes have no adverse effects, and the Food and Nutrition Board urges people to be cautious about consuming excessive amounts of riboflavin.[9]
Function
Flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD) function as cofactors for a variety of flavoprotein enzyme reactions:
- Flavoproteins of electron transport chain, including FMN in Complex I and FAD in Complex II
- FAD is required for the production of pyridoxic acid from pyridoxal (vitamin B6) by pyridoxine 5'-phosphate oxidase
- The primary coenzyme form of vitamin B6 (pyridoxal phosphate) is FMN dependent
- Oxidation of pyruvate, α-ketoglutarate, and branched-chain amino acids requires FAD in the shared E3 portion of their respective dehydrogenase complexes
- Fatty acyl CoA dehydrogenase requires FAD in fatty acid oxidation
- FAD is required to convert retinol (vitamin A) to retinoic acid via cytosolic retinal dehydrogenase
- Synthesis of an active form of folate (5-methyltetrahydrofolate) from 5,10-methylenetetrahydrofolate by methylenetetrahydrofolate reductase is FADH2 dependent
- FAD is required to convert tryptophan to niacin (vitamin B3)
- Reduction of the oxidized form of glutathione (GSSG) to its reduced form (GSH) by glutathione reductase is FAD dependent
For the molecular mechanism of action see main articles flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD)
Dietary recommendations
| United States | ||
| Age group (years) | RDA for riboflavin (mg/d)[9] | Tolerable upper intake level[9] |
|---|---|---|
| Infants 0–6 months | 0.3* | ND |
| Infants 6–12 months | 0.4* | |
| 1–3 | 0.5 | |
| 4–8 | 0.6 | |
| 9–13 | 0.9 | |
| Females 14–18 | 1.0 | |
| Males 14–18 | 1.3 | |
| Females 19+ | 1.1 | |
| Males 19+ | 1.3 | |
| Pregnant females 14–50 | 1.4 | |
| Lactating females 14–50 | 1.6 | |
| European Food Safety Authority | ||
| Age group (years) | Adequate Intake of riboflavin (mg/d)[20] | Tolerable upper limit[20] |
| 7–11 months | 0.4 | ND |
| 1–3 | 0.6 | |
| 4–6 | 0.7 | |
| 7–10 | 1.0 | |
| 11–14 | 1.4 | |
| 15–17 | 1.6 | |
| 18+ | ||
| Australia and New Zealand | ||
| Age group (years) | Adequate Intake of riboflavin (mg/d)[21] | Upper level of intake[21] |
| 0–6 months | 0.3* | ND |
| 7–12 months | 0.4* | |
| 1–3 | 0.5 | |
| 4–8 | 0.6 | |
| 9–13 | 0.9 | |
| Females 14–70 | 1.1 | |
| Males 14–70 | 1.3 | |
| Females >70 | 1.3 | |
| Males >70 | 1.6 | |
| Pregnant females 14–50 | 1.4 | |
| Lactating females 14–50 | 1.6 | |
| * Adequate intake for infants, no RDA/RDI yet established[9] | ||
The National Academy of Medicine (then the U.S. Institute of Medicine [IOM]) updated Estimated Average Requirements (EARs) and Recommended Dietary Allowances (RDAs) for riboflavin in 1998. The current EARs for riboflavin for women and men ages 14 and up are 0.9 mg/day and 1.1 mg/day, respectively; the RDAs are 1.1 and 1.3 mg/day, respectively. RDAs are higher than EARs so as to identify amounts that will cover people with higher than average requirements. RDA for pregnancy is 1.4 mg/day. RDA for lactation is 1.6 mg/day. For infants up to 12 months the Adequate Intake (AI) is 0.3–0.4 mg/day. and for children ages 1–13 years the RDA increases with age from 0.5 to 0.9 mg/day. As for safety, the IOM sets Tolerable upper intake levels (ULs) for vitamins and minerals when evidence is sufficient. In the case of riboflavin there is no UL, as there is no human data for adverse effects from high doses. Collectively the EARs, RDAs, AIs and ULs are referred to as Dietary Reference Intakes (DRIs).[17][9]
The European Food Safety Authority (EFSA) refers to the collective set of information as Dietary Reference Values, with Population Reference Intake (PRI) instead of RDA, and Average Requirement instead of EAR. AI and UL defined the same as in United States. For women and men ages 15 and older the PRI is set at 1.6 mg/day. PRI for pregnancy is 1.9 mg/day, for lactation 2.0 mg/day. For children ages 1–14 years the PRIs increase with age from 0.6 to 1.4 mg/day. These PRIs are higher than the U.S. RDAs.[22] The EFSA also reviewed the safety question and like the U.S., decided that there was not sufficient information to set an UL.[23]
For U.S. food and dietary supplement labeling purposes the amount in a serving is expressed as a percent of Daily Value (%DV). For riboflavin labeling purposes 100% of the Daily Value was 1.7 mg, but as of May 27, 2016, it was revised to 1.3 mg to bring it into agreement with the RDA.[24][25] Compliance with the updated labeling regulations was required by 1 January 2020, for manufacturers with $10 million or more in annual food sales, and by 1 January 2021 for manufacturers with less than $10 million in annual food sales.[26][27][28] During the first six months following the 1 January 2020 compliance date, the FDA plans to work cooperatively with manufacturers to meet the new Nutrition Facts label requirements and will not focus on enforcement actions regarding these requirements during that time.[26] A table of the old and new adult Daily Values is provided at Reference Daily Intake.
Sources
Food and beverages that provide riboflavin without fortification are milk, cheese, eggs, leaf vegetables, liver, kidneys, lean meats, legumes, mushrooms, and almonds.[9][10]
The milling of cereals results in considerable loss (up to 60%) of vitamin B2, so white flour is enriched in some countries by addition of the vitamin. The enrichment of bread and ready-to-eat breakfast cereals contributes significantly to the dietary supply of vitamin B2. Polished rice is not usually enriched, because the vitamin's yellow color would make the rice visually unacceptable to the major rice-consuming populations. However, most of the flavin content of whole brown rice is retained if the rice is steamed (parboiled) prior to milling. This process drives the flavins in the germ and aleurone layers into the endosperm. Free riboflavin is naturally present in foods along with protein-bound FMN and FAD. Bovine milk contains mainly free riboflavin, with a minor contribution from FMN and FAD. In whole milk, 14% of the flavins are bound noncovalently to specific proteins.[29] Milk and yogurt contain some of the highest riboflavin content.[4] Egg white and egg yolk contain specialized riboflavin-binding proteins, which are required for storage of free riboflavin in the egg for use by the developing embryo.
Riboflavin is added to baby foods, breakfast cereals, pastas and vitamin-enriched meal replacement products. It is difficult to incorporate riboflavin into liquid products because it has poor solubility in water, hence the requirement for riboflavin-5'-phosphate (E101a), a more soluble form of riboflavin. Riboflavin is also used as a food coloring and as such is designated in Europe as the E number E101.[30]
Chemistry
As a chemical compound, riboflavin is a yellow-orange solid substance with poor solubility in water compared to other B vitamins. Visually, it imparts color to vitamin supplements (and bright yellow color of urine in persons taking it).[2]
Industrial uses
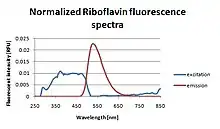
Because riboflavin is fluorescent under UV light, dilute solutions (0.015–0.025% w/w) are often used to detect leaks or to demonstrate coverage in an industrial system such a chemical blend tank or bioreactor.
Industrial synthesis

The industrial scale production of riboflavin using diverse microorganisms, including filamentous fungi such as Ashbya gossypii, Candida famata and Candida flaveri, as well as the bacteria Corynebacterium ammoniagenes and Bacillus subtilis.[31] The latter organism, genetically modified to both increase the production of riboflavin and to introduce an antibiotic (ampicillin) resistance marker, is employed at a commercial scale to produce riboflavin for feed and food fortification. The chemical company BASF has installed a plant in South Korea, which is specialized on riboflavin production using Ashbya gossypii. The concentrations of riboflavin in their modified strain are so high that the mycelium has a reddish/brownish color and accumulates riboflavin crystals in the vacuoles, which will eventually burst the mycelium. Riboflavin is sometimes overproduced, possibly as a protective mechanism, by some bacteria in the presence of high concentrations of hydrocarbons or aromatic compounds. One such organism is Micrococcus luteus (American Type Culture Collection strain number ATCC 49442), which develops a yellow color due to production of riboflavin while growing on pyridine, but not when grown on other substrates, such as succinic acid.[32]
History
The name "riboflavin" comes from "ribose" (the sugar whose reduced form, ribitol, forms part of its structure) and "flavin", the ring-moiety which imparts the yellow color to the oxidized molecule (from Latin flavus, "yellow").[33] The reduced form, which occurs in metabolism along with the oxidized form, is colorless.
"Vitamin B" was originally considered to have two components, a heat-labile vitamin B1 and a heat-stable vitamin B2.[1] In the 1920s, vitamin B2 was initially thought to be the factor necessary for preventing pellagra.[1] In 1923, Paul Gyorgy in Heidelberg was investigating egg-white injury in rats;[1] the curative factor for this condition was called vitamin H, which is now called biotin. Since both pellagra and vitamin H deficiency were associated with dermatitis, Gyorgy decided to test the effect of vitamin B2 on vitamin H deficiency in rats. He enlisted the service of Wagner-Jauregg in Kuhn's laboratory.[1] In 1933, Kuhn, Gyorgy, and Wagner found that thiamin-free extracts of yeast, liver, or rice bran prevented the growth failure of rats fed a thiamin-supplemented diet.[1]
Further, the researchers noted that a yellow-green fluorescence in each extract promoted rat growth, and that the intensity of fluorescence was proportional to the effect on growth.[1] This observation enabled them to develop a rapid chemical and bioassay to isolate the factor from egg white in 1933.[1] The same group then isolated the same preparation (a growth-promoting compound with yellow-green fluorescence) from whey using the same procedure (lactoflavin). In 1934, Kuhn's group identified the structure of so-called flavin and synthesized vitamin B2, leading to evidence in 1939 that riboflavin was essential for human health.[1]
Research
Donated whole blood can be treated with riboflavin and then with ultraviolet light as a pathogen reduction technology.[34]
Other animals
In other animals, riboflavin deficiency results in lack of growth,[35] failure to thrive, and eventual death. Experimental riboflavin deficiency in dogs results in growth failure, weakness, ataxia, and inability to stand. The animals collapse, become comatose, and die. During the deficiency state, dermatitis develops together with hair loss. Other signs include corneal opacity, lenticular cataracts, hemorrhagic adrenals, fatty degeneration of the kidney and liver, and inflammation of the mucous membrane of the gastrointestinal tract.[36] Post-mortem studies in rhesus monkeys fed a riboflavin-deficient diet revealed about one-third the normal amount of riboflavin was present in the liver, which is the main storage organ for riboflavin in mammals.[37] Riboflavin deficiency in birds results in low egg hatch rates.[38]
See also
- Flavin
- Riboflavin kinase
- Riboflavin synthase
References
- 1 2 3 4 5 6 7 8 9 Northrop-Clewes CA, Thurnham DI (2012). "The discovery and characterization of riboflavin". Annals of Nutrition & Metabolism. 61 (3): 224–30. doi:10.1159/000343111. PMID 23183293. Archived from the original on 29 August 2021. Retrieved 7 November 2018.
- 1 2 3 4 5 6 7 8 9 "Riboflavin". Drugs.com, The American Society of Health-System Pharmacists. 1 August 2018. Archived from the original on 30 December 2016. Retrieved 7 November 2018.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 13 June 2020. Retrieved 15 September 2020.
- 1 2 3 4 5 6 7 "Riboflavin: Fact Sheet for Health Professionals". Office of Dietary Supplements, US National Institutes of Health. 20 August 2018. Archived from the original on 24 February 2020. Retrieved 7 November 2018.
- ↑ "Why fortify?". Food Fortification Initiative. 2017. Archived from the original on 4 April 2017. Retrieved 4 April 2017.
- ↑ Squires, Victor R. (2011). The Role of Food, Agriculture, Forestry and Fisheries in Human Nutrition – Volume IV. EOLSS Publications. p. 121. ISBN 9781848261952. Archived from the original on 25 December 2020. Retrieved 8 February 2019.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- 1 2 Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 230. ISBN 9781284057560.
- 1 2 3 4 5 6 7 8 Institute of Medicine (1998). "Riboflavin". Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: The National Academies Press. pp. 87–122. ISBN 978-0-309-06554-2. Archived from the original on 17 July 2015. Retrieved 29 August 2017.
- 1 2 Higdon, Jane; Drake, Victoria J. (2007). "Riboflavin". Micronutrient Information Center. Linus Pauling Institute at Oregon State University. Archived from the original on 11 February 2010. Retrieved 3 December 2009.
- ↑ Merrill AH, McCormick DB (2020). "Riboflavin". In BP Marriott, DF Birt, VA Stallings, AA Yates (eds.). Present Knowledge in Nutrition, Eleventh Edition. London, United Kingdom: Academic Press (Elsevier). pp. 189–208. ISBN 978-0-323-66162-1.
- ↑ "Map: Count of Nutrients In Fortification Standards". Global Fortification Data Exchange. Archived from the original on 11 April 2019. Retrieved 1 August 2020.
- ↑ Mastropasqua L (2015). "Collagen cross-linking: when and how? A review of the state of the art of the technique and new perspectives". Eye and Vision. 2: 19. doi:10.1186/s40662-015-0030-6. PMC 4675057. PMID 26665102.
- ↑ Thompson DF, Saluja HS (August 2017). "Prophylaxis of migraine headaches with riboflavin: A systematic review". Journal of Clinical Pharmacy and Therapeutics. 42 (4): 394–403. doi:10.1111/jcpt.12548. PMID 28485121.
- ↑ McCormick, DB (2012). "Riboflavin". In JW Erdman Jr; IA MacDonald; SH Zeisel (eds.). Present Knowledge in Nutrition (Tenth ed.). Hoboken, NJ: Wiley-Blackwell. pp. 280–92. ISBN 978-0-470-95917-6.
- 1 2 "Riboflavin (Vitamin B2)". Archived from the original on 6 July 2020. Retrieved 4 July 2020.
- 1 2 Gropper SS, Smith JL, Groff JL (2009). "Ch. 9: Riboflavin". Advanced Nutrition and Human Metabolism (5th ed.). Wadsworth: CENGAG Learning. pp. 329–33. ISBN 9780495116578.
- ↑ Boehnke C, Reuter U, Flach U, Schuh-Hofer S, Einhäupl KM, Arnold G (July 2004). "High-dose riboflavin treatment is efficacious in migraine prophylaxis: an open study in a tertiary care centre". European Journal of Neurology. 11 (7): 475–7. doi:10.1111/j.1468-1331.2004.00813.x. PMID 15257686.
- ↑ Zempleni J, Galloway JR, McCormick DB (January 1996). "Pharmacokinetics of orally and intravenously administered riboflavin in healthy humans". The American Journal of Clinical Nutrition. 63 (1): 54–66. doi:10.1093/ajcn/63.1.54. PMID 8604671.
- 1 2 European Food Safety Authority (February 2006). "Tolerable Upper Intake Levels for Vitamins and Minerals" (PDF). EFSA. Archived (PDF) from the original on 16 March 2016. Retrieved 18 June 2018.
- 1 2 "Nutrient reference values for Australia and New Zealand" (PDF). National Health and Medical Research Council. 9 September 2005. Archived from the original (PDF) on 21 January 2017. Retrieved 19 June 2018.
- ↑ "Overview on Dietary Reference Values for the EU population as derived by the EFSA Panel on Dietetic Products, Nutrition and Allergies" (PDF). 2017. Archived (PDF) from the original on 28 August 2017.
- ↑ "Tolerable Upper Intake Levels For Vitamins And Minerals" (PDF). European Food Safety Authority. 2006. Archived (PDF) from the original on 16 March 2016.
- ↑ "Federal Register May 27, 2016 Food Labeling: Revision of the Nutrition and Supplement Facts Labels. FR page 33982" (PDF). Archived (PDF) from the original on 8 August 2016.
- ↑ "Daily Value Reference of the Dietary Supplement Label Database (DSLD)". Dietary Supplement Label Database (DSLD). Archived from the original on 7 April 2020. Retrieved 16 May 2020.
- 1 2 "FDA provides information about dual columns on Nutrition Facts label". U.S. Food and Drug Administration (FDA). 30 December 2019. Archived from the original on 23 January 2021. Retrieved 16 May 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Changes to the Nutrition Facts Label". U.S. Food and Drug Administration (FDA). 27 May 2016. Archived from the original on 6 May 2018. Retrieved 16 May 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Industry Resources on the Changes to the Nutrition Facts Label". U.S. Food and Drug Administration (FDA). 21 December 2018. Archived from the original on 25 December 2020. Retrieved 16 May 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ Kanno C, Kanehara N, Shirafuji K, Tanji R, Imai T (February 1991). "Binding form of vitamin B2 in bovine milk: its concentration, distribution and binding linkage". Journal of Nutritional Science and Vitaminology. 37 (1): 15–27. doi:10.3177/jnsv.37.15. PMID 1880629.
- ↑ "Current EU approved additives and their E Numbers". UK Food Standards Agency. 27 July 2007. Archived from the original on 7 October 2010. Retrieved 3 December 2009.
- ↑ Stahmann KP, Revuelta JL, Seulberger H (May 2000). "Three biotechnical processes using Ashbya gossypii, Candida famata, or Bacillus subtilis compete with chemical riboflavin production". Applied Microbiology and Biotechnology. 53 (5): 509–516. doi:10.1007/s002530051649. PMID 10855708.
- ↑ Sims GK, O'loughlin EJ (October 1992). "Riboflavin Production during Growth of Micrococcus luteus on Pyridine". Applied and Environmental Microbiology. 58 (10): 3423–3425. PMC 183117. PMID 16348793.
- ↑ "Riboflavin". Online Etymology Dictionary, Douglas Harper. 2018. Archived from the original on 8 November 2018. Retrieved 7 November 2018.
- ↑ Yonemura S, Doane S, Keil S, Goodrich R, Pidcoke H, Cardoso M (July 2017). "Improving the safety of whole blood-derived transfusion products with a riboflavin-based pathogen reduction technology". Blood Transfusion = Trasfusione del Sangue. 15 (4): 357–364. doi:10.2450/2017.0320-16. PMC 5490732. PMID 28665269Note: Authored by Terumo employees
{{cite journal}}: CS1 maint: postscript (link) - ↑ Patterson BE, Bates CJ (May 1989). "Riboflavin deficiency, metabolic rate and brown adipose tissue function in sucking and weanling rats". The British Journal of Nutrition. 61 (3): 475–483. doi:10.1079/bjn19890137. PMID 2547428.
- ↑ Sebrell WH, Onstott RH (1938). "Riboflavin Deficiency in Dogs". Public Health Reports. 53 (3): 83–94. doi:10.2307/4582435. JSTOR 4582435.
- ↑ Waisman, Harry A. (1944). "Production of Riboflavin Deficiency in the Monkey". Experimental Biology and Medicine. 55 (1): 69–71. doi:10.3181/00379727-55-14462.
- ↑ Romanoff AL, Bauernfeind JC (1942). "Influence of riboflavin-deficiency in eggs on embryonic development (Gallus domesticus)". The Anatomical Record. 82 (1): 11–23. doi:10.1002/ar.1090820103.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- Higdon, Jane, "Riboflavin", Micronutrient Information Center, Linus Pauling Institute, Oregon State University