Vitamin K epoxide reductase
| Vitamin K epoxide reductase (warfarin-sensitive) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
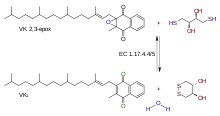 Reaction | |||||||||
| Identifiers | |||||||||
| EC no. | 1.17.4.4 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| Vitamin K epoxide reductase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
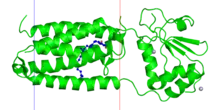 Structure of a bacterial VKOR, membrane denoted as lines (PDB: 3KP9). | |||||||||
| Identifiers | |||||||||
| Symbol | VKOR | ||||||||
| Pfam | PF07884 | ||||||||
| InterPro | IPR012932 | ||||||||
| CATH | 3kp9 | ||||||||
| TCDB | 9.B.265 | ||||||||
| OPM superfamily | 18 | ||||||||
| OPM protein | 3kp9 | ||||||||
| |||||||||
Vitamin K epoxide reductase (VKOR) is an enzyme (EC 1.17.4.4) that reduces vitamin K after it has been oxidised in the carboxylation of glutamic acid residues in blood coagulation enzymes. VKOR is a member of a large family of predicted enzymes that are present in vertebrates, Drosophila, plants, bacteria and archaea.[1] In some plant and bacterial homologues, the VKOR domain is fused with domains of the thioredoxin family of oxidoreductases.[1]
Four cysteine residues and one residue, which is either serine or threonine, are identified as likely active-site residues.[1] Solved bacterial VKOR structures has enabled more insights into the catalytic mechanism. All VKORs are transmembrane proteins with at least three TM helices at the catalytic core. The quinone to be reduced is bound by a redox-active CXXC motif in the C-terminal helices, similar to the DsbB active site. Two other cysteines to the N-terminal are located in a loop outside of the transmembrane region; they relay electrons with a redox protein (or in the case of the bacterial homolog, its own fused domain).[2][3]
The human gene for VKOR is called VKORC1 (VKOR complex subunit 1). It is the target of anticoagulant warfarin. Its partner is a redox protein with an unknown identity.[4][5] There is also a similar gene called VKORC1L1.
References
- 1 2 3 Goodstadt L, Ponting CP (June 2004). "Vitamin K epoxide reductase: homology, active site and catalytic mechanism". Trends in Biochemical Sciences. 29 (6): 289–92. doi:10.1016/j.tibs.2004.04.004. PMID 15276181.
- ↑ Li W, Schulman S, Dutton RJ, Boyd D, Beckwith J, Rapoport TA (January 2010). "Structure of a bacterial homologue of vitamin K epoxide reductase". Nature. 463 (7280): 507–12. Bibcode:2010Natur.463..507L. doi:10.1038/nature08720. PMC 2919313. PMID 20110994.
- ↑ Rishavy MA, Usubalieva A, Hallgren KW, Berkner KL (March 2011). "Novel insight into the mechanism of the vitamin K oxidoreductase (VKOR): electron relay through Cys43 and Cys51 reduces VKOR to allow vitamin K reduction and facilitation of vitamin K-dependent protein carboxylation". The Journal of Biological Chemistry. 286 (9): 7267–78. doi:10.1074/jbc.M110.172213. PMC 3044983. PMID 20978134.
- ↑ Li T, Chang CY, Jin DY, Lin PJ, Khvorova A, Stafford DW (February 2004). "Identification of the gene for vitamin K epoxide reductase". Nature. 427 (6974): 541–4. Bibcode:2004Natur.427..541L. doi:10.1038/nature02254. PMID 14765195. S2CID 4424554.
- ↑ Rost S, Fregin A, Ivaskevicius V, Conzelmann E, Hörtnagel K, Pelz HJ, Lappegard K, Seifried E, Scharrer I, Tuddenham EG, Müller CR, Strom TM, Oldenburg J (February 2004). "Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2". Nature. 427 (6974): 537–41. Bibcode:2004Natur.427..537R. doi:10.1038/nature02214. PMID 14765194. S2CID 4424197.