Ifenprodil
 | |
| Names | |
|---|---|
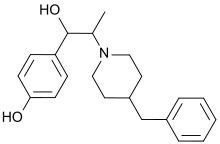
| IUPAC name
4-[2-(4-Benzylpiperidin-1-yl)-1-hydroxypropyl]phenol | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.041.341 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C21H27NO2 |
| Molar mass | 325.445 |
| Pharmacology | |
| C04AX28 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ifenprodil is an inhibitor of the NMDA receptor,[1] specifically of GluN1 (glycine-binding NMDA receptor subunit 1) and GluN2B (glutamate-binding NMDA receptor subunit 2) subunits.[2] Additionally, ifenprodil inhibits GIRK channels, and interacts with alpha1 adrenergic, serotonin, and sigma receptors.[3]
NMDA receptors are multimeric ionotropic glutamate receptors composed of four subunits. GluN1 is obligate for functional expression. Other subunits include GluN2A, GluN2B, and the more recently discovered GluN3 subunits. Ifenprodil selectively inhibits NMDA receptors containing the GluN2B subunit.
As ifenprodil tartrate, it has been marketed in some countries, including Japan and France, as a cerebral vasodilator, under the trade names Cerocral, Dilvax, and Vadilex.[4]
It is currently in phase III clinical trials to treat SARS-CoV2 infection and phase II trials for idiopathic pulmonary fibrosis.[5]
References
- ↑ Reynolds IJ, Miller RJ (1989). "Ifenprodil is a novel type of N-methyl-D-aspartate receptor antagonist: interaction with polyamines". Mol. Pharmacol. 36 (5): 758–65. PMID 2555674.
- ↑ Korinek M, Kapras V, Vyklicky V, Adamusova E, Borovska J, Vales K, Stuchlik A, Horak M, Chodounska H, Vyklicky L Jr (Dec 2011). "Neurosteroid modulation of N-methyl-d-aspartate receptors: Molecular mechanism and behavioral effects". Steroids. 76 (13): 1409–18. doi:10.1016/j.steroids.2011.09.002. PMID 21925193. S2CID 195681796.
- ↑ Kobayashi, Toru; Washiyama, Kazuo; Ikeda, Kazutaka (3 August 2005). "Inhibition of G Protein-Activated Inwardly Rectifying K+ Channels by Ifenprodil". Neuropsychopharmacology. 31 (3): 516–524. doi:10.1038/sj.npp.1300844. PMID 16123769.
- ↑ The Merck Index (Thirteenth Edition). Whitehouse Station, NJ: Merck Research Laboratories Division of Merck & Co., Inc. 2001. p. 878.
- ↑ "Ifenprodil - Algernon Pharmaceuticals - AdisInsight". adisinsight.springer.com. Retrieved March 25, 2021.