Mibampator
 | |
| Clinical data | |
|---|---|
| Other names | LY-451395 |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
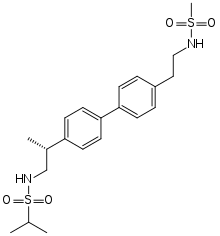
| Formula | C21H30N2O4S2 |
| Molar mass | 438.60 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Mibampator (developmental code name LY-451395) is a positive allosteric modulator (PAM) of the AMPA receptor (AMPAR), an ionotropic glutamate receptor, which was under development by Eli Lilly for the treatment of agitation/aggression in Alzheimer's disease but was never marketed.[1][2] It reached phase II clinical trials prior to the discontinuation of its development.[1]
Mibampator belongs to the biarylpropylsulfonamide group of AMPAR PAMs, which also includes LY-404187, LY-503430, and PF-04958242 among others.[3] It is a "high-impact" AMPAR potentiator, unlike "low-impact" AMPAR potentiators from other classes like CX-516 and its congener farampator (CX-691, ORG-24448), and is able to elicit comparatively more robust increases in AMPAR signaling.[2] In animals, high-impact AMPAR potentiators enhance cognition and memory at low doses, but produce motor coordination disruptions, convulsions, and neurotoxicity at higher doses.[4]
Mibampator failed to produce cognitive improvement in patients with Alzheimer's disease, though it did show improvements in neuropsychiatric measures.[5] A caveat of the study was that the maximally tolerated dosage of the drug could not be used due to toxicity, and dosages in the same range in rodents notably failed to improve memory-related behavior.[6]
See also
References
- 1 2 "Mibampator - AdisInsight".
- 1 2 Roberts BM, Holden DE, Shaffer CL, Seymour PA, Menniti FS, Schmidt CJ, Williams GV, Castner SA (2010). "Prevention of ketamine-induced working memory impairments by AMPA potentiators in a nonhuman primate model of cognitive dysfunction". Behav. Brain Res. 212 (1): 41–8. doi:10.1016/j.bbr.2010.03.039. PMID 20347881. S2CID 9432930.
- ↑ Froestl W, Muhs A, Pfeifer A (2012). "Cognitive enhancers (nootropics). Part 1: drugs interacting with receptors". J. Alzheimers Dis. 32 (4): 793–887. doi:10.3233/JAD-2012-121186. PMID 22886028.
- ↑ Ranganathan M, DeMartinis N, Huguenel B, Gaudreault F, Bednar MM, Shaffer CL, Gupta S, Cahill J, Sherif MA, Mancuso J, Zumpano L, D'Souza DC (2017). "Attenuation of ketamine-induced impairment in verbal learning and memory in healthy volunteers by the AMPA receptor potentiator PF-04958242". Mol. Psychiatry. 22 (11): 1633–1640. doi:10.1038/mp.2017.6. PMID 28242871. S2CID 3691566.
- ↑ Zarate CA, Manji HK (2008). "The role of AMPA receptor modulation in the treatment of neuropsychiatric diseases". Exp. Neurol. 211 (1): 7–10. doi:10.1016/j.expneurol.2008.01.011. PMC 2441819. PMID 18291371.
- ↑ Buccafusco JJ (2009). "Emerging cognitive enhancing drugs". Expert Opin Emerg Drugs. 14 (4): 577–89. doi:10.1517/14728210903257796. PMID 19772371. S2CID 20980837.
External links