Farampator
 | |
 | |
| Clinical data | |
|---|---|
| Other names | CX-691; ORG-24448 |
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
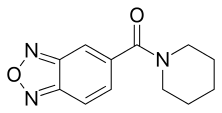
| Formula | C12H13N3O2 |
| Molar mass | 231.255 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Farampator (developmental code names CX-691, ORG-24448, SCH-900460) is an ampakine drug. It was developed by Cortex Pharmaceuticals, and licensed to Organon BioSciences for commercial development. Following the purchase of Organon by Schering-Plough in 2007, the development license to farampator was transferred. The development of farampator was eventually terminated, reportedly due to concerns about cardiac toxicity.[1][2]
Farampator has been investigated for its effect on AMPA receptors and researched for potential use in the treatment of schizophrenia and Alzheimer's disease. It was found to improve short-term memory, but impaired episodic memory. It produced side effects such as headache, somnolence and nausea. Subjects reporting side effects had significantly higher plasma levels of farampator than subjects without. Additional analyses revealed that in the farampator condition the group without side effects showed a significantly superior memory performance relative to the group with side effects.[3]
See also
References
- ↑ "Farampator - AdisInsight".
- ↑ Froestl W, Muhs A, Pfeifer A (2012). "Cognitive enhancers (nootropics). Part 1: drugs interacting with receptors". J. Alzheimers Dis. 32 (4): 793–887. doi:10.3233/JAD-2012-121186. PMID 22886028.
- ↑ Wezenberg E, Verkes RJ, Ruigt GS, Hulstijn W, Sabbe BG (Jun 2007). "Acute effects of the ampakine farampator on memory and information processing in healthy elderly volunteers". Neuropsychopharmacology. 32 (6): 1272–83. doi:10.1038/sj.npp.1301257. PMID 17119538.