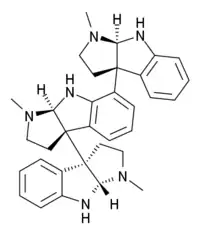
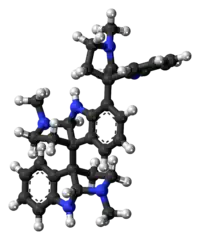
Hodgkinsine
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C33H38N6 |
| Molar mass | 518.709 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
| | |
Hodgkinsine is an alkaloid found in plants of the genus Psychotria, particularly Psychotria colorata,[1] although it is also found in Psychotria lyciiflora[2] and probably other species in this family,[3]
Hodgkinsine has antiviral, antibacterial and antifungal effects, but has mainly been researched for the analgesic effects that it produces, and is thought to be one of the components responsible for the analgesic effects seen when Psychotria colorata is used in traditional medical practice in humans. It has been found to act as both a mu opioid agonist and an NMDA antagonist,[4] both of which are mechanisms of action shared with commonly used painkillers (morphine and ketamine respectively, and which occur concurrently in the clinical analgesics tramadol and levorphanol).
Hodgkinsine is a trimer composed of three pyrrolidinoindoline subunits, with the monomer closely resembling another alkaloid eseroline which has similar bioactivity. Due to its complex structure and multiple chiral centres, hodgkinsine has many stereoisomers and significant research has been undertaken to elucidate the structure-activity relationships of the various isomers and synthetic derivatives structurally derived from hodgkinsine.[5][6][7]
See also
References
- ↑ Verotta L, Pilati T, Tatò M, Elisabetsky E, Amador TA, Nunes DS (March 1998). "Pyrrolidinoindoline Alkaloids from Psychotria colorata1". Journal of Natural Products. 61 (3): 392–6. doi:10.1021/np9701642. PMID 9548883.
- ↑ Jannic V, Guéritte F, Laprévote O, Serani L, Martin MT, Sévenet T, Potier P (June 1999). "Pyrrolidinoindoline alkaloids from Psychotria oleoides and Psychotria lyciiflora". Journal of Natural Products. 62 (6): 838–43. doi:10.1021/np9805387. PMID 10395499.
- ↑ Saad HE, el-Sharkawy SH, Shier WT (August 1995). "Biological activities of pyrrolidinoindoline alkaloids from Calycodendron milnei". Planta Medica. 61 (4): 313–6. doi:10.1055/s-2006-958090. PMID 7480176.
- ↑ Amador TA, Verotta L, Nunes DS, Elisabetsky E (December 2000). "Antinociceptive profile of hodgkinsine". Planta Medica. 66 (8): 770–2. doi:10.1055/s-2000-9604. PMID 11199142.
- ↑ Verotta L, Orsini F, Sbacchi M, Scheildler MA, Amador TA, Elisabetsky E (July 2002). "Synthesis and antinociceptive activity of chimonanthines and pyrrolidinoindoline-type alkaloids". Bioorganic & Medicinal Chemistry. 10 (7): 2133–42. doi:10.1016/s0968-0896(02)00078-0. PMID 11983509.
- ↑ Kodanko JJ, Overman LE (June 2003). "Enantioselective total syntheses of the cyclotryptamine alkaloids hodgkinsine and hodgkinsine B". Angewandte Chemie. 42 (22): 2528–31. doi:10.1002/anie.200351261. PMID 12800178.
- ↑ Kodanko JJ, Hiebert S, Peterson EA, Sung L, Overman LE, de Moura Linck V, et al. (October 2007). "Synthesis of all low-energy stereoisomers of the tris(pyrrolidinoindoline) alkaloid hodgkinsine and preliminary assessment of their antinociceptive activity". The Journal of Organic Chemistry. 72 (21): 7909–14. doi:10.1021/jo7013643. PMID 17887704.