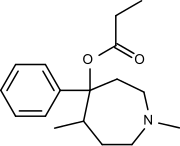
Proheptazine
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ECHA InfoCard | 100.000.916 |
| Chemical and physical data | |
| Formula | C17H25NO2 |
| Molar mass | 275.392 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Proheptazine is an opioid analgesic from the phenazepine family. It was invented in the 1960s.[1]
Proheptazine produces similar effects to other opioids, including analgesia, sedation, euphoria, dizziness and nausea.
In the United States it is a Schedule I Narcotic controlled substance with an ACSCN of 9643 and a 2013 annual aggregate manufacturing quota of zero. The salts in use are the citrate (free base conversion ratio 0.589), hydrobromide (0.773), and hydrochloride (0.883).[2][3]
References
- ↑ Diamond J, Bruce WF, Tyson FT (January 1964). "Synthesis and Properties of the Analgesic DL-α-1,3-dimethyl-4-phenyl-4-propionoxyazacycloheptane (Proheptazine)". Journal of Medicinal Chemistry. 7: 57–60. doi:10.1021/jm00331a013. PMID 14186026.
- ↑ "Quotas - 2014". Diversion Control Division. Drug Enforcement Agency, U.S. Department of Justice.
- ↑ "Conversion Factors for Controlled Substances". Diversion Control Division. Drug Enforcement Agency, U.S. Department of Justice.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.