Norbuprenorphine
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.208.387 |
| Chemical and physical data | |
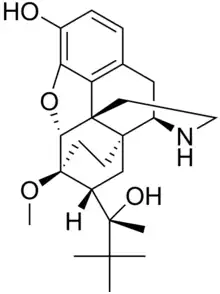
| Formula | C25H35NO4 |
| Molar mass | 413.558 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Norbuprenorphine is a major active metabolite of the opioid modulator buprenorphine. It is a μ-opioid, δ-opioid, and nociceptin receptor full agonist,[1][2] and a κ-opioid receptor partial agonist.[2] In rats, unlike buprenorphine, norbuprenorphine produces marked respiratory depression but with very little antinociceptive effect.[3] In explanation of these properties, norbuprenorphine has been found to be a high affinity P-glycoprotein substrate, and in accordance, shows very limited blood-brain-barrier penetration.[3]
Norbuprenorhine
See also
- Norbuprenorphine-3-glucuronide
- Buprenorphine-3-glucuronide
- Loperamide
- Noroxymorphone
References
- ↑ Yassen A, Kan J, Olofsen E, Suidgeest E, Dahan A, Danhof M (May 2007). "Pharmacokinetic-pharmacodynamic modeling of the respiratory depressant effect of norbuprenorphine in rats". The Journal of Pharmacology and Experimental Therapeutics. 321 (2): 598–607. doi:10.1124/jpet.106.115972. PMID 17283225. S2CID 11921738.
- 1 2 Huang P, Kehner GB, Cowan A, Liu-Chen LY (May 2001). "Comparison of pharmacological activities of buprenorphine and norbuprenorphine: norbuprenorphine is a potent opioid agonist". The Journal of Pharmacology and Experimental Therapeutics. 297 (2): 688–95. PMID 11303059.
- 1 2 Brown SM, Campbell SD, Crafford A, Regina KJ, Holtzman MJ, Kharasch ED (October 2012). "P-glycoprotein is a major determinant of norbuprenorphine brain exposure and antinociception". J. Pharmacol. Exp. Ther. 343 (1): 53–61. doi:10.1124/jpet.112.193433. PMC 3464040. PMID 22739506.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.