HS665
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
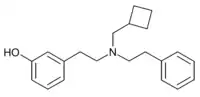
| Formula | C21H27NO |
| Molar mass | 309.453 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
HS665 is a drug which acts as a potent and selective κ-opioid receptor agonist, and has analgesic effects in animal studies.[1][2][3][4]
References
- ↑ Spetea M, Berzetei-Gurske IP, Guerrieri E, Schmidhammer H (November 2012). "Discovery and pharmacological evaluation of a diphenethylamine derivative (HS665), a highly potent and selective κ opioid receptor agonist". Journal of Medicinal Chemistry. 55 (22): 10302–6. doi:10.1021/jm301258w. PMID 23134120.
- ↑ Spetea M, Eans SO, Ganno ML, Lantero A, Mairegger M, Toll L, et al. (August 2017). "Selective κ receptor partial agonist HS666 produces potent antinociception without inducing aversion after i.c.v. administration in mice". British Journal of Pharmacology. 174 (15): 2444–2456. doi:10.1111/bph.13854. PMID 28494108.
- ↑ Erli F, Guerrieri E, Ben Haddou T, Lantero A, Mairegger M, Schmidhammer H, Spetea M (September 2017). "Highly Potent and Selective New Diphenethylamines Interacting with the κ-Opioid Receptor: Synthesis, Pharmacology, and Structure-Activity Relationships". Journal of Medicinal Chemistry. 60 (17): 7579–7590. doi:10.1021/acs.jmedchem.7b00981. PMID 28825813.
- ↑ Zhu L, Cui Z, Zhu Q, Zha X, Xu Y. "Novel Opioid Receptor Agonists with Reduced Morphine-like Side Effects". Mini Reviews in Medicinal Chemistry. 18 (19): 1603–1610. doi:10.2174/1389557518666180716124336. PMID 30009707.
Opioid receptor modulators | |
|---|---|
| MOR |
|
| DOR |
|
| KOR |
|
| NOP |
|
| Unsorted |
|
| Others |
|
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.