Hemorphin-4
 | |
| Names | |
|---|---|
| IUPAC name
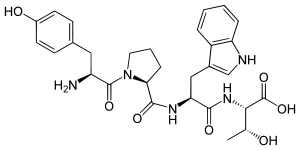
(2S,3R)-2-[[(2S)-2-[[(2S)-1-[(2S)-2-amino-3-(4-hydroxyphenyl)propanoyl]pyrrolidine-2-carbonyl]amino]-3-(1H-indol-3-yl)propanoyl]amino]-3-hydroxybutanoic acid | |
| Other names
L-tyrosyl-L-prolyl-L-tryptophyl-L-threonine | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C29H35N5O7 |
| Molar mass | 565.618 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hemorphin-4 is an endogenous opioid peptide of the hemorphin family which possesses antinociceptive properties and is derived from the β-chain of hemoglobin in the bloodstream.[1][2] It is a tetrapeptide with the amino acid sequence Tyr-Pro-Trp-Thr. Hemorphin-4 has affinities for the μ-, δ-, and κ-opioid receptors that are in the same range as the structurally related β-casomorphins, although affinity to the κ-opioid receptor is markedly higher in comparison.[3] It acts as an agonist at these sites.[4] Hemorphin-4 also has inhibitory effects on angiotensin-converting enzyme (ACE),[5] and as a result, may play a role in the regulation of blood pressure.[3] Notably, inhibition of ACE also reduces enkephalin catabolism.[6]
See also
- Casomorphin
References
- ↑ Brantl V, Gramsch C, Lottspeich F, Mertz R, Jaeger KH, Herz A (June 1986). "Novel opioid peptides derived from hemoglobin: hemorphins". European Journal of Pharmacology. 125 (2): 309–10. doi:10.1016/0014-2999(86)90044-0. PMID 3743640.
- ↑ Davis TP, Gillespie TJ, Porreca F (1989). "Peptide fragments derived from the beta-chain of hemoglobin (hemorphins) are centrally active in vivo". Peptides. 10 (4): 747–51. doi:10.1016/0196-9781(89)90107-1. PMID 2587417. S2CID 11168006.
- 1 2 Liebmann C, Schrader U, Brantl V (August 1989). "Opioid receptor affinities of the blood-derived tetrapeptides hemorphin and cytochrophin". European Journal of Pharmacology. 166 (3): 523–6. doi:10.1016/0014-2999(89)90368-3. PMID 2553436.
- ↑ Erchegyi J, Kastin AJ, Zadina JE (1992). "Isolation of a novel tetrapeptide with opiate and antiopiate activity from human brain cortex: Tyr-Pro-Trp-Gly-NH2 (Tyr-W-MIF-1)". Peptides. 13 (4): 623–31. doi:10.1016/0196-9781(92)90165-Y. PMID 1359507. S2CID 32330624.
- ↑ Lantz I, Glämsta EL, Talbäck L, Nyberg F (August 1991). "Hemorphins derived from hemoglobin have an inhibitory action on angiotensin converting enzyme activity". FEBS Letters. 287 (1–2): 39–41. doi:10.1016/0014-5793(91)80011-Q. PMID 1652464. S2CID 26892050.
- ↑ Benuck M, Berg MJ, Marks N (1982). "Separate metabolic pathways for Leu-enkephalin and Met-enkephalin-Arg(6)-Phe(7) degradation by rat striatal synaptosomal membranes". Neurochemistry International. 4 (5): 389–96. doi:10.1016/0197-0186(82)90081-X. PMID 20487892. S2CID 23138078.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.