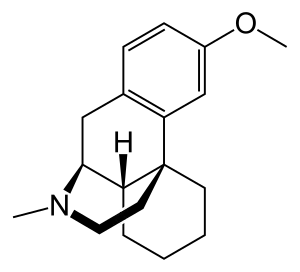
Methorphan
Levomethorphan (L), dextromethorphan (R) | |||
| Clinical data | |||
|---|---|---|---|
| ATC code |
| ||
| Legal status | |||
| Legal status |
| ||
| Identifiers | |||
IUPAC name
| |||
| CAS Number |
| ||
| PubChem CID | |||
| ChemSpider |
| ||
| UNII | |||
| ChEBI | |||
| ChEMBL | |||
| Chemical and physical data | |||
| Formula | C18H25NO | ||
| Molar mass | 271.404 g·mol−1 | ||
| 3D model (JSmol) | |||
SMILES
| |||
InChI
| |||
| | |||
Methorphan comes in two isomeric forms, each with differing pharmacology and effects:
- Dextromethorphan - An over-the-counter cough suppressant, as well as dissociative hallucinogen.
- Levomethorphan - A potent opioid analgesic that was never clinically developed; a prodrug of the powerful opioid agonist analgesic levorphanol (Levo-Dromoran).
Racemethorphan refers to the racemic mixture of both of these stereoisomers. It is listed under the Single Convention on Narcotic Drugs 1961 and is therefore listed in the United States as a Controlled Substance, specifically as a Narcotic in Schedule II with an ACSCN of 9732 and an annual aggregate manufacturing quota of 3 grams in 2014.[1] The salts in use are the hydrobromide (free base conversion ratio 0.770) and the tartrate (0.644)
See also
References
- ↑ "Conversion Factors for Controlled Substances". www.deadiversion.usdoj.gov.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.