Diclofensine
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
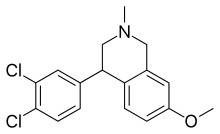
| Formula | C17H17Cl2NO |
| Molar mass | 322.23 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Diclofensine (Ro 8-4650) was developed by Hoffmann-La Roche in the 1970s[1] in the search for a new antidepressant. It was found that the (S)-isomer was responsible for activity.[2] Is a stimulant drug which acts as a triple monoamine reuptake inhibitor,[3][4] primarily inhibiting the reuptake of dopamine[5] and norepinephrine,[6] with affinities (Ki) of 16.8 nM, 15.7 nM, and 51 nM for DAT, NET, and SERT (dopamine, norepinephrine and serotonin transporters), respectively.[7] It was found to be an effective antidepressant in human trials,[8][9][10] with relatively few side effects,[11] but was ultimately dropped from clinical development, possibly due to concerns about its abuse potential.[12][13]
Diclofensine is chemically a tetrahydroisoquinoline (THIQ) derivative, as is nomifensine.
See also
References
- ↑ US Patent 3947456 A, Alfred Rheiner, "Substituted 4-Phenyl Isoquinolines", published 1976-03-30, assigned to Hoffman-La Roche Inc.
- ↑ Crossley, Roger (1995). Chirality and the Biological Activity of Drugs. 2000 Corporate Blvd., N.W., Boca Raton, Florida 33431: CRC Press, Inc. p. 138. ISBN 978-0-8493-9140-8.
{{cite book}}: CS1 maint: location (link) - ↑ Keller HH, Schaffner R, Carruba MO, Burkard WP, Pieri M, Bonetti EP, et al. (1982). "Diclofensine (Ro 8-4650)--a potent inhibitor of monoamine uptake: biochemical and behavioural effects in comparison with nomifensine". Advances in Biochemical Psychopharmacology. 31: 249–63. PMID 6979165.
- ↑ Omer LM (July 1982). "Pilot trials with diclofensine, a new psychoactive drug in depressed patients". International Journal of Clinical Pharmacology, Therapy, and Toxicology. 20 (7): 320–6. PMID 7107085.
- ↑ Di Renzo G, Amoroso S, Taglialatela M, Canzoniero LM, Maida P, Lombardi G, Annunziato L (1988). "Pure uptake blockers of dopamine can reduce prolactin secretion: studies with diclofensine". Life Sciences. 42 (21): 2161–9. doi:10.1016/0024-3205(88)90131-2. PMID 2968488.
- ↑ Gasić S, Korn A, Eichler HG (May 1986). "Effect of diclofensine, a novel antidepressant, on peripheral adrenergic function". Clinical Pharmacology and Therapeutics. 39 (5): 582–5. doi:10.1038/clpt.1986.100. PMID 3698467. S2CID 34977195.
- ↑ Andersen PH (August 1989). "The dopamine inhibitor GBR 12909: selectivity and molecular mechanism of action". European Journal of Pharmacology. 166 (3): 493–504. doi:10.1016/0014-2999(89)90363-4. PMID 2530094.
- ↑ Cherpillod C, Omer LM (1981). "A controlled trial with diclofensine, a new psychoactive drug, in the treatment of depression". The Journal of International Medical Research. 9 (5): 324–9. doi:10.1177/030006058100900505. PMID 7028532. S2CID 41618656.
- ↑ Omer OL, Díaz-Olivera M, Ismail S (March 1984). "Therapeutic efficacy and tolerance of diclofensine in psychoreactive depression--a double-blind comparison with placebo". Methods and Findings in Experimental and Clinical Pharmacology. 6 (3): 147–51. PMID 6379345.
- ↑ Funke HJ, Holtmann W, Ismail S, Jansen W, Leonhardt KF, Muth H, et al. (May 1986). "Double-blind comparison of diclofensine with nomifensine in outpatients with dysphoric mood". Pharmacopsychiatry. 19 (3): 120–3. doi:10.1055/s-2007-1017168. PMID 3725890.
- ↑ Culig J, Ehsanullah RS, Hallett C, Iliopoulou A, Matheson I, Turner P (May 1983). "A clinical pharmacological comparison of diclofensine (Ro 8-4650) with nomifensine and amitriptyline in normal human volunteers". British Journal of Clinical Pharmacology. 15 (5): 537–43. doi:10.1111/j.1365-2125.1983.tb02087.x. PMC 1427714. PMID 6860528.
- ↑ Lamb RJ, Griffiths RR (1990). "Self-administration in baboons and the discriminative stimulus effects in rats of bupropion, nomifensine, diclofensine and imipramine". Psychopharmacology. 102 (2): 183–90. doi:10.1007/bf02245920. PMID 2125734. S2CID 25043692.
- ↑ Nakachi N, Kiuchi Y, Inagaki M, Inazu M, Yamazaki Y, Oguchi K (August 1995). "Effects of various dopamine uptake inhibitors on striatal extracellular dopamine levels and behaviours in rats". European Journal of Pharmacology. 281 (2): 195–203. doi:10.1016/0014-2999(95)00246-h. PMID 7589207.