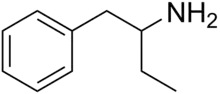
Phenylisobutylamine
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C10H15N |
| Molar mass | 149.237 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Phenylisobutylamine, also known as α-ethylphenethylamine, Butanphenamine, B or AEPEA,[1] is a stimulant drug of the phenethylamine class. It is a higher homologue of amphetamine, differing from amphetamine's molecular structure only by the substitution of the methyl group at the alpha position of the side chain with an ethyl group. Compared to amphetamine, phenylisobutylamine has strongly reduced dopaminergic effects, and instead acts as a selective norepinephrine releasing agent. The dextroisomer of phenylisobutylamine partially substitutes for dextroamphetamine in rats.[1]
A number of derivatives of phenylisobutylamine are known, including BDB, MBDB, EBDB, butylone (bk-MBDB), eutylone (bk-EBDB), Ariadne (α-Et-DOM), 4-CAB, and 4-MAB.
"Phenylisobutylamine" is in fact a chemical misnomer because isobutylamine itself contains a branched chain. The correct name after this style for this class of compound would be "phenylsecbutylamine".
See also
References
- 1 2 Oberlender R, Nichols DE (1991). "Structural variation and (+)-amphetamine-like discriminative stimulus properties". Pharmacol Biochem Behav. 38 (3): 581–586. doi:10.1016/0091-3057(91)90017-V. PMID 2068194. S2CID 19069907.
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|