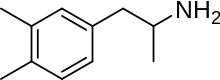
Xylopropamine
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider |
|
| UNII |
|
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C11H17N |
| Molar mass | 163.264 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
SMILES
| |
InChI
| |
| | |
Xylopropamine (Perhedrin, Esanin), also known as 3,4-dimethylamphetamine, is a stimulant drug of the phenethylamine and amphetamine classes which was developed and marketed as an appetite suppressant in the 1950s.[1]
Xylopropamine was briefly sold as the sulfate salt, but it was not widely marketed. Other related amphetamine derivatives such as 2,4-dimethylamphetamine were also investigated for the same purpose, however these drugs had negative side effects such as high blood pressure and were not very successful, mainly due to the introduction of alternative drugs like phentermine which had similar efficacy but fewer side effects.
Xylopropamine was also reported as having analgesic[2] and anti-inflammatory[3] effects but its side effect profile resulted in it never being further developed for these applications.
See also
- Methamphetamine
- Dimethylamphetamine
- 3,4-Dimethylmethcathinone
- 4-Methylamphetamine
- 4-Methylmethamphetamine
- Indanylaminopropane
References
- ↑ US 2384700, Schnider O, "Alkylated phenyl-isopropyl-amines and process for the manufacture of same", issued 11 September 1945, assigned to Hoffman La Roche
- ↑ Harris SC, Worley RC (June 1957). "Analgesic properties of xylopropamine". Proceedings of the Society for Experimental Biology and Medicine. 95 (2): 212–5. doi:10.3181/00379727-95-23170. PMID 13441687. S2CID 34027366.
- ↑ Randall LO, Selitto JJ, Valdes J (December 1957). "Anti-inflammatory effects of xylopropamine". Archives Internationales de Pharmacodynamie et de Therapie. 113 (1–2): 233–49. PMID 13509788.
| DRAs |
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NRAs |
| ||||||||||||||
| SRAs |
| ||||||||||||||
| Others |
| ||||||||||||||
See also: Receptor/signaling modulators • Monoamine reuptake inhibitors • Adrenergics • Dopaminergics • Serotonergics • Monoamine metabolism modulators • Monoamine neurotoxins | |||||||||||||||
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|