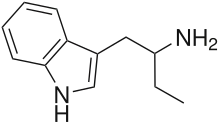
Α-Ethyltryptamine
 | |
 | |
| Clinical data | |
|---|---|
| Other names | alpha-Ethyltryptamine; αET; AET; Etryptamine; 3-(2-Aminobutyl)indole; 3-Indolylbutylamine; Ro 3-1932; NSC-88061 |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| PubChem SID | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C12H16N2 |
| Molar mass | 188.274 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 222 to 223 °C (432 to 433 °F) |
SMILES
| |
InChI
| |
| (verify) | |
α-Ethyltryptamine (αET, AET), also known as etryptamine (INN, BAN, USAN), is a psychedelic, stimulant, and entactogenic drug of the tryptamine class.[2][3] It was originally developed and marketed as an antidepressant under the brand name Monase by Upjohn in the 1960s.[4]
History
Originally believed to exert its effects predominantly via monoamine oxidase inhibition, α-ethyltryptamine was developed during the 1960s as an antidepressant by Upjohn chemical company in the United States under the name Monase, but was withdrawn from potential commercial use due to incidence of idiosyncratic agranulocytosis.[5]
α-Ethyltryptamine gained limited recreational popularity as a designer drug in the 1980s. Subsequently, in the USA it was added to the Schedule I list of illegal substances in 1993.
Pharmacology
α-Ethyltryptamine is structurally and pharmacologically related to αMT, α-methyltryptamine, and it is believed[5] its central stimulant activity is probably not due to its activity as an MAOI, but appears to stem from its structural relationship to the indolic psychedelics. In contrast to αMT, α-ethyltryptamine is less stimulating and hallucinogenic, its effects resembling more those of entactogens like MDMA ("Ecstasy").
Similarly to αMT, α-ethyltryptamine is a releasing agent of serotonin, norepinephrine and dopamine, with serotonin being the primary neurotransmitter affected.[6] In addition, it acts as a non-selective serotonin receptor agonist. A study performed in 1991[7] with rat subjects provided evidence that α-ethyltryptamine may induce serotonergic neurotoxicity similar to that of MDMA. As with many other serotonin releasing agents, injury can occur when excessive doses are taken or when combined with drugs such as other MAOIs.[8]
References
- ↑ Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- ↑ ""Erowid AET (alpha-ethyltryptamine) Vault"".
- ↑ Alexander Shulgin; Ann Shulgin. "#11, a-ET: Alpha-Ethyltryptamine; Indole,3-(2-Aminobutyl); Tryptamine,Alpha-Ethyl; 3-(2-Aminobutyl)Indole; Monase,". "Tryptamines i Have Known And Loved: The Continuation", Part 2, "The Chemistry Continues". Erowid Online Library. p. 11. Retrieved 15 November 2013.
- ↑ US Patent 3296072, Szmuszkovicz Jacob, "Method of Treating Mental Depression", published 1967-01-03, assigned to Upjohn Co
- 1 2 Alexander Shulgin; Ann Shulgin (1997). ""Part 2, The Chemistry Continues: #11, a-ET: Alpha-Ethyltryptamine; Indole,3-(2-Aminobutyl); Tryptamine,Alpha-Ethyl; 3-(2-Aminobutyl)Indole; Monase," part v, "EXTENSIONS AND COMMENTARY."" (Book). Tryptamines i Have Known and Loved: The Continuation, (1st. ed.). Berkeley, CA : Transform Press, ©1997. ISBN 978-0-9630096-9-2. Retrieved 15 November 2013.
This base, a-ET or etryptamine, was a promising anti-depressant, explored clinically as the acetate salt by Upjohn under the name of Monase. Its central stimulant activity is probably not due to its monoamineoxidase inhibition activity, but appears to stem from its structural relationship to the indolic psychedelics. It was withdrawn from potential commercial use with the appearance of an unacceptable incidence of a medical condition known as agranulocytosis, but the extra mural research into its action, among the lay population, goes on,
- ↑ Blough BE, Landavazo A, Partilla JS, Decker AM, Page KM, Baumann MH, Rothman RB (October 2014). "Alpha-ethyltryptamines as dual dopamine-serotonin releasers". Bioorganic & Medicinal Chemistry Letters. 24 (19): 4754–4758. doi:10.1016/j.bmcl.2014.07.062. PMC 4211607. PMID 25193229.
- ↑ Huang XM, Johnson MP, Nichols DE (July 1991). "Reduction in brain serotonin markers by alpha-ethyltryptamine (Monase)". European Journal of Pharmacology. 200 (1): 187–190. doi:10.1016/0014-2999(91)90686-K. PMID 1722753.
- ↑ Gillman PK (October 2005). "Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity". British Journal of Anaesthesia. 95 (4): 434–441. doi:10.1093/bja/aei210. PMID 16051647. "Drugs such as MDMA, ecstasy (3,4-methylenedioxymethamphetamine), if combined with MAOIs (including moclobemide) do also cause fatalities because they act as serotonin releasers,"
External links
Empathogens/entactogens | |
|---|---|
| Phenylalkyl- amines (other than cathinones) |
|
| Cyclized phenyl- alkylamines | |
| Cathinones |
|
| Tryptamines | |
| Chemical classes | |
| Adamantanes | |
|---|---|
| Adenosine antagonists |
|
| Alkylamines | |
| Ampakines | |
| Arylcyclohexylamines | |
| Benzazepines | |
| Cathinones |
|
| Cholinergics |
|
| Convulsants | |
| Eugeroics |
|
| Oxazolines | |
| Phenethylamines |
|
| Phenylmorpholines |
|
| Piperazines | |
| Piperidines |
|
| Pyrrolidines | |
| Racetams | |
| Tropanes |
|
| Tryptamines |
|
| Others |
|
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| DRAsTooltip Dopamine releasing agents |
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NRAsTooltip Norepinephrine releasing agents |
| ||||||||||||||
| SRAsTooltip Serotonin releasing agents |
| ||||||||||||||
| Others |
| ||||||||||||||
See also: Receptor/signaling modulators • Monoamine reuptake inhibitors • Adrenergics • Dopaminergics • Serotonergics • Monoamine metabolism modulators • Monoamine neurotoxins | |||||||||||||||
| Adrenergic |
|
|---|---|
| Dopaminergic |
|
| Serotonergic |
|
See also: Receptor/signaling modulators • Adrenergics • Dopaminergics • Melatonergics • Serotonergics • Monoamine reuptake inhibitors • Monoamine releasing agents • Monoamine metabolism modulators | |
|