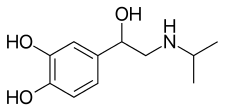
Isoprenaline
 | |
| Names | |
|---|---|
| Trade names | Isoprenaline Macure, others[1] |
| Other names | Isoproterenol (USAN US) |
IUPAC name
| |
| Clinical data | |
| Drug class | β adrenoceptor agonist (non-selective)[2] |
| Main uses | Slow heart rate, heart block, shock, bronchospasm[3] |
| Side effects | Headache, anxiety, blurry vision, palpitations, chest pain, sweating, tremor[3] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Inhalation (80–120 μg), intravenous injection (IV) |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601236 |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Elimination half-life | ~2 minutes |
| Chemical and physical data | |
| Formula | C11H17NO3 |
| Molar mass | 211.261 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Isoprenaline, also known as isoproterenol, is a medication which has been used to treat slow heart rate, heart block, shock, and bronchospasm.[3] In shock, norepinephrine is generally preferred.[3] It is generally given by injection.[3]
Common side effects include headache, anxiety, blurry vision, palpitations, chest pain, sweating, and tremor.[3] It should not be used in people with cardiac glycoside toxicity.[3] Use in pregnancy appears safe.[4] It is a non-selective β adrenoceptor agonist.[2]
Isoprenaline was approved for medical use in the US in 1947.[5] In the United States 0.2 mg for injection costs about 190 USD as of 2021.[6] By mouth and inhaled forms are no longer available commercially in the United States.[3] It is available under various brand names.[1]
Medical uses
It is used to treat heart block and episodes of Adams-Stokes syndrome that are not caused by ventricular tachycardia or fibrillation, in emergencies for cardiac arrest until electric shock can be administered, for bronchospasm occurring during anesthesia, and as an adjunct in the treatment of hypovolemic shock, septic shock, low cardiac output (hypoperfusion) states, congestive heart failure, and cardiogenic shock.[2]
Historically, it was used to treat asthma via metered aerosol or nebulizing devices; it was also available in sublingual, oral, intravenous, and intramuscular formulations.[5] The U.S. National Asthma Education and Prevention Program Expert Panel recommends against its use as a nebulizer for acute bronchoconstriction.[7]
Isoprenaline can also ameliorate the impairment of intestinal stem cells mediated by β2-adrenoreceptors after chemotherapy.[8]
Dosage
For bronchospasm it may be given at a dose of 0.01 to 0.02 mg by injection into a vein.[2]
Contraindications
It should not be used in people with tachyarrhythmias, tachycardia or heart block caused by digitalis poisoning, ventricular arrhythmias which require inotropic therapy, or with angina.[2]
Side effects
Side effects of isoprenaline include nervousness, headache, dizziness, nausea, visual blurring, tachycardia, palpitations, angina, Adams-Stokes attacks, pulmonary edema, hypertension, hypotension, ventricular arrhythmias, tachyarrhythmias, difficulty breathing, sweating, mild tremors, weakness, flushing, and pallor.[2] Isoproterenol has been reported to cause insulin resistance leading to diabetic ketoacidosis.[9]
Pharmacology
The adverse effects of isoprenaline are also related to the drug's cardiovascular effects. Isoprenaline can produce tachycardia (an elevated heart rate), which predisposes people who take it to cardiac arrhythmias.[5]
Pharmacodynamics
Isoprenaline is a β1 and β2 adrenoreceptor agonist and has almost no activity on alpha adrenergic receptors.[5] Its agonist effects at TAAR1 provide it with pharmacodynamic effects that resemble those of the endogenous trace amines, like tyramine.[10]
Isoprenaline's effects on the cardiovascular system (non-selective) relate to its actions on cardiac β1 receptors and β2 receptors on smooth muscle within the tunica media of arterioles. Isoprenaline has positive inotropic and chronotropic effects on the heart. β2 adrenoceptor stimulation in arteriolar smooth muscle induces vasodilation. Its inotropic and chronotropic effects elevate systolic blood pressure, while its vasodilatory effects tend to lower diastolic blood pressure. The overall effect is to decrease mean arterial pressure due to the β2 receptors' vasodilation.[11]
The isopropylamine group in isoprenaline makes it selective for β receptors. The free catechol hydroxyl groups keep it susceptible to enzymatic metabolism.[12]
Pharmacokinetics
The plasma half-life for isoprenaline is approximately two minutes.
Chemistry
It is structurally related to epinephrine.[2]
History
It was first approved in the US in 1947.[5] Between 1963 and 1968 in England, Wales, Scotland, Ireland, Australia, and New Zealand there was an increase in deaths among people using isoprenaline to treat asthma. This was attributed to overdose: the inhalers produced in that area were dispensing five times the dosage dispensed by inhalers produced in the US and Canada, where the deaths were not observed.[13][14]
Society and culture
Brands

As of June 2017, isoprenaline was marketed under many brand names worldwide and as two different salts: Aleudrina, Asthpul, Iludrin, Isomenyl, Isoprenalin, Isoprenalina, Isoprenalina, Isoprenalina, Isoprenaline, Isoprenaline Macure, Isoprénaline, Isoprénaline, Isoprenaline hydrochloride, Isoprenaline sulfate, Isoprenalinesulfaat, Isoprenalinsulfat, Isoprenalinum, Isopropydine, Isopropylnoradrenaline, Isoproterenol, Isoproterenol, Isoproterenol, Isoproterenol hydrochloride, Isoproterenol sulfate, Isuprel, Isuprel, Neo-Epinine, Neodrenal, Proternol, Saventrine, and Win 5162.[1] It is also marketed as a combination drug with cromoglicic acid as Frenal Compositum, in combination with pronase as Isopal P, and in combination with atropine as Stmerin D.[1]
References
- 1 2 3 4 "Isoprenaline international brands". Drugs.com. Archived from the original on 26 June 2019. Retrieved 21 June 2017.
- 1 2 3 4 5 6 7 "Label: Isoproterenol hydrochloride injection, solution". NIH DailyMed. September 10, 2013. Archived from the original on 11 January 2017. Retrieved 21 June 2017.
- 1 2 3 4 5 6 7 8 "Isoproterenol Monograph for Professionals". Drugs.com. Archived from the original on 28 January 2021. Retrieved 28 November 2021.
- ↑ "Isoproterenol Use During Pregnancy". Drugs.com. Archived from the original on 28 November 2020. Retrieved 28 November 2021.
- 1 2 3 4 5 Mozayani, Ashraf; Raymon, Lionel (2003). Handbook of Drug Interactions: A Clinical and Forensic Guide. Springer Science & Business Media. pp. 541–542. ISBN 9781592596546. Archived from the original on 2019-12-15. Retrieved 2021-10-18.
- ↑ "Isoproterenol Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 27 January 2021. Retrieved 28 November 2021.
- ↑ National Asthma Education and Prevention Program Expert Panel (August 28, 2007). "Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma" (PDF). NIH National Heart, Lung, and Blood Institute. Archived (PDF) from the original on June 26, 2019. Retrieved October 18, 2021.
- ↑ Zeng H, Li H, Yue M, et al. Isoprenaline protects intestinal stem cells from chemotherapy-induced damage. British Journal of Pharmacology. 2020 Feb;177(3):687-700. DOI: 10.1111/bph.14883
- ↑ Hoff, R; Koh, CK (2018). "Isoproterenol Induced Insulin Resistance Leading to Diabetic Ketoacidosis in Type 1 Diabetes Mellitus". Case Reports in Endocrinology. 2018: 4328954. doi:10.1155/2018/4328954. PMC 6311779. PMID 30647979.
- ↑ Kleinau G, Pratzka J, Nürnberg D, Grüters A, Führer-Sakel D, Krude H, Köhrle J, Schöneberg T, Biebermann H (October 2011). "Differential modulation of Beta-adrenergic receptor signaling by trace amine-associated receptor 1 agonists". PLOS ONE. 6 (10): e27073. Bibcode:2011PLoSO...627073K. doi:10.1371/journal.pone.0027073. PMC 3205048. PMID 22073124.
"Table 1: EC50 values of different agonists at hTAAR1, hADRB1 and hADRB2. Archived 2018-09-20 at the Wayback Machine" - ↑ Korbut, Ryszard (2017). Farmakologia (in polski). Wydawnictwo Lekarskie PZWL. p. 36. ISBN 9788320053685.
- ↑ Mehta, Akul (January 27, 2011). "Notes - Medicinal Chemistry of the Peripheral Nervous System - Adrenergics and Cholinergic". Pharmaxchange. Archived from the original on 4 November 2010. Retrieved 21 June 2017.
- ↑ Pierce, Neil; Hensley, Michael J. (1998). "Epidemiologic Studies of Beta Agonists and Asthma Deaths". Epidemiologic Studies. 20 (2): 173–86. doi:10.1093/oxfordjournals.epirev.a017979. PMID 9919437.
- ↑ Jalba, MS (2008). "Three generations of ongoing controversies concerning the use of short acting beta-agonist therapy in asthma: a review". The Journal of Asthma. 45 (1): 9–18. doi:10.1080/02770900701495512. PMID 18259990. S2CID 31732029.
External links
| Identifiers: |
|---|