Zileuton
 | |
 | |
| Names | |
|---|---|
| Trade names | Zyflo, other |
IUPAC name
| |
| Clinical data | |
| Drug class | Leukotriene modifier[1] |
| Main uses | Asthma[1] |
| Side effects | Nausea, sore throat, liver problems[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697013 |
| Legal | |
| License data | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | Not yet established |
| Protein binding | 93% |
| Metabolism | Liver (CYP1A2, CYP2C9 and CYP3A4-mediated) |
| Elimination half-life | 2.5 hours |
| Excretion | Kidney |
| Chemical and physical data | |
| Formula | C11H12N2O2S |
| Molar mass | 236.29 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
SMILES
| |
InChI
| |
Zileuton, sold under the trade name Zyflo among others, is a medication used for the maintenance treatment of asthma.[1] It may be used as an add on therapy when inhaled steroids are insufficient.[1] It is taken by mouth two to four times per day.[1]
Common side effects include nausea and a sore throat.[1] Other side effects may include liver problems.[1] It is unclear if use during pregnancy is safe, with some animal studies finding harm.[2] It is a leukotriene modifier that blocks the production of leukotrienes.[1]
Zileuton was approved for medical use in the United States in 1996.[1] It is not approved in Europe.[3] It is available as a generic medication.[4] A month of medication in the United States costs about 900 USD as of 2021.[4] It is available in an immediate and extended release formulation.[1]
Medical uses

Zileuton is used for the long term treatment of asthma in adults and children 12 years of age and older. It is not indicated for use in the reversal of bronchospasm in acute asthma attacks. Therapy with zileuton can be continued during acute exacerbations of asthma.
Dosage
The recommended dose of immediate release is one 600 mg four times per day.[1] The recommended dose of extended release is two 600 mg tablets twice daily.[1]
Related compounds include montelukast and zafirlukast. These two compounds are leukotriene receptor antagonists which block the action of specific leukotrienes, while zileuton inhibits leukotriene formation.
Contraindications
The most serious side effect is a potential elevation of liver enzymes (in 2% of people). Therefore, zileuton is contraindicated in active liver disease or persistent hepatic function enzymes elevations greater than three times the upper limit of normal. Hepatic function should be assessed prior to initiating Zyflo CR, monthly for the first 3 months, every 2–3 months for the remainder of the first year, and periodically thereafter.
Neuropsychiatric events, including sleep disorders and behavioral changes, may occur with Zyflo and Zyflo CR. Patients should be instructed to notify their healthcare provider if neuropsychiatric events occur while using the medication.
Zileuton is a weak inhibitor of CYP1A2[5] and thus has three clinically important drug interactions, which include increasing theophylline, and propranolol levels. It has been shown to lower theophylline clearance significantly, doubling the AUC and prolonging half-life by nearly 25%. Because of theophylline's relation to caffeine (both being a methylxanthine, and theophylline being a metabolite of caffeine), caffeine's metabolism and clearance may also be reduced, but there are no drug interaction studies between zileuton and caffeine.[6] The R-isomer of warfarin metabolism and clearance is mainly affected by zileuton, while the S-isomer is not (because of metabolism via different enzymes). This can lead to an increase in prothrombin time.[7]
Side effects
The most common adverse reactions reported by patients treated with Zyflo CR were sinusitis (6.5%), nausea (5%), and pharyngolaryngeal pain (5%) vs. placebo, 4%, 1.5%, and 4% respectively.
Interactions
Drug interactions
Zileuton is a minor substrate of CYP1A2, 2C8/9, 3A4, and a weak inhibitor of CYP 1A2. The drug has been shown to increase the serum concentration or effects of theophylline, propranolol, and warfarin, although significant increase in prothrombin time is not obvious. It is advised that the doses of each medication be monitored and/or reduced accordingly.
Other interactions
The avoidance of alcohol is recommended due to increase risk of CNS depression as well as an increase risk in liver toxicity. In addition, the herbal supplement St. John's wort may decrease the serum levels of zileuton.[8]
Overdose
Symptoms
Human experience of acute overdose with zileuton is limited. A patient in a clinical study took between 6.6 and 9.0 grams of zileuton immediate-release tablets in a single dose. Vomiting was inducted and the patient recovered without sequelae. Zileuton is not removed by dialysis.
The oral minimum lethal doses in mice and rats were 500-4000 and 300–1000 mg/kg, respectively (providing greater than 3 and 9 times the systemic exposure (AUC) achieved at the maximum recommended human daily oral dose, respectively). In dogs, at an oral dose of 1000 mg/kg (providing in excess of 12 times the systemic exposure (AUC) achieved at the maximum recommended human daily oral dose) no deaths occurred but nephritis was reported.
Treatment
Should an overdose occur, the patient should be treated symptomatically and supportive measures instituted as required. If indicated, elimination of unabsorbed drug should be achieved by emesis or gastric lavage; usual precautions should be observed to maintain the airway.
Chemistry
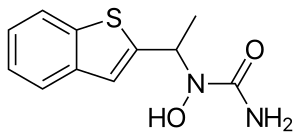
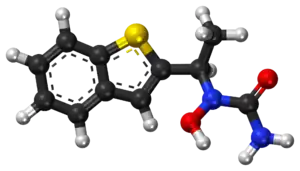
Zileuton is an active oral inhibitor of the enzyme 5-lipoxygenase, which forms leukotrienes, 5-hydroxyeicosatetraenoic acid, and 5-oxo-eicosatetraenoic acid from arachidonic acid. The chemical name of zileuton is (±)-1-(1-Benzo[b]thien-2-ylethyl)-l-hydroxyurea.[9]
The molecular formula of zileuton is C11H12N2O2S with a molecular weight of 236.29. The formulation from the manufacturer is a racemic mixture of R(+) and S(-) enantiomers.[10]
Pharmacokinetics
Following oral administration zileuton is rapidly absorbed with a mean time to peak blood serum concentration of 1.7 hours and an average half-life elimination of 2.5 hours. Blood plasma concentrations are proportional to dose, whereas the absolute bioavailability is unknown.
The apparent volume of distribution of zileuton is approximately 1.2 L/kg. Zileuton is 93% bound to plasma proteins, primarily to albumin, with minor binding to alpha-1-acid glycoprotein.
Elimination of zileuton is primarily through metabolites in the urine (~95%) with the feces accounting for the next largest amount (~2%). The drug is metabolized by the cytochrome P450 enzymes: CYP1A2, 2C9, and 3A4.[10]
See also
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 "Zileuton Monograph for Professionals". Drugs.com. Archived from the original on 27 August 2019. Retrieved 4 August 2021.
- 1 2 "Zileuton Use During Pregnancy". Drugs.com. Archived from the original on 18 January 2021. Retrieved 4 August 2021.
- ↑ Talbot, John; Waller, Patrick (19 November 2004). Stephens' Detection of New Adverse Drug Reactions. John Wiley & Sons. p. 151. ISBN 978-0-470-09265-1. Archived from the original on 27 August 2021. Retrieved 4 August 2021.
- 1 2 "Zileuton ER Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 13 June 2018. Retrieved 4 August 2021.
- ↑ Lu P, Schrag ML, Slaughter DE, Raab CE, Shou M, Rodrigues AD (November 2003). "Mechanism-based inhibition of human liver microsomal cytochrome P450 1A2 by zileuton, a 5-lipoxygenase inhibitor". Drug Metabolism and Disposition. 31 (11): 1352–60. doi:10.1124/dmd.31.11.1352. PMID 14570767. S2CID 22714315. Archived from the original on 2021-08-27. Retrieved 2021-07-10.
- ↑ Cafcit (caffeine citrate) package insert. Evansville, IN: Mead Johnson & Company; 2003 May.
- ↑ Zyflo Filmtab (zileuton) package insert. Chicago, IL: Abbott Laboratories; 1998 Mar.
- ↑ Zileuton monograph. Lexi-Comp Online, Lexi-Drugs Online, Lexi-Comp Inc. Hudson, OH. Available at: "Archived copy". Archived from the original on November 16, 2004. Retrieved September 14, 2008.
{{cite web}}: CS1 maint: archived copy as title (link). Accessed November 12th, 2008. - ↑ Krutetskaya, Z. I.; Milenina, L. S.; Naumova, A. A.; Antonov, V. G.; Nozdrachev, A. D. (2016). "5-Lipoxygenase inhibitor zileuton inhibits Ca2+-responses induced by glutoxim and molixan in macrophages". Doklady Biochemistry and Biophysics. 469 (1): 302–304. doi:10.1134/S1607672916040177. ISSN 1607-6729. PMID 27599517. S2CID 3132863.
- 1 2 "Zyflo Drug Information". Archived from the original on 2021-04-20. Retrieved 2021-07-10.
External links
| Identifiers: |
|---|
- Zyflo Archived 2009-02-02 at the Wayback Machine (patient information)
- https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm093664.htm Archived 2016-05-10 at the Wayback Machine