Olodaterol
 | |
| Names | |
|---|---|
| Pronunciation | Striverdi Respimat /ˈstrɪvərdi ˈrɛspɪmæt/ STRIV-ər-dee RES-pim-at |
| Trade names | Striverdi Respimat |
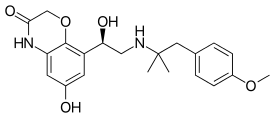
IUPAC name
| |
| Clinical data | |
| Drug class | LABA[1] |
| Main uses | COPD[1] |
| Side effects | Cough, diarrhea, joint pain, rash, runny nose[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Inhalation (MDI) |
| Typical dose | 5 ucg/day[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | ~30% (inhalation)[2] |
| Protein binding | ~60% |
| Metabolism | Liver |
| Elimination half-life | 7.5 hours |
| Excretion | Feces (53%), urine (38%) — following IV administration |
| Chemical and physical data | |
| Formula | C21H26N2O5 |
| Molar mass | 386.448 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Olodaterol, sold under the brand name Striverdi, is a long-acting β adrenoreceptor agonist (LABA) used to treat chronic obstructive pulmonary disease (COPD).[1] Use is not recommended in asthma.[1] It is breathed into the lungs.[1]
Common side effects include cough, diarrhea, joint pain, rash, and runny nose.[1] Other side effects may include QT prolongation and bronchospasm.[1] In asthma there are concerns regarding an increased risk of death.[1] While there is no evidence of harm in pregnancy, such use has not been well studied.[3]
Olodaterol was approved for medical use in the United States in 2014.[1] It is also available in combination with tiotropium as a single inhaler.[1] In the United Kingdom a month of medication costs the NHS about £26 as of 2021.[4] In the United States this amount costs about 230 USD.[5]
Medical uses
Olodaterol is a once-daily maintenance bronchodilator treatment of airflow obstruction in people with COPD.[6] While it appears to reduce COPD exacerbations it does not appear to alter the speed at which a person's lungs worsen or alter their life expectancy.[6]
As of December 2013, olodaterol is not approved for the treatment of asthma.
Dosage
It is used at a dose of 5 mcg once per day.[1] It is administered in an inhaler called Respimat Soft Mist Inhaler.
Side effects
Side effects generally were rare and mild in clinical studies. Most common, but still affecting no more than 1% of patients, were nasopharyngitis (running nose), dizziness and rash. To judge from the drug's mechanism of action and from experiences with related drugs, hypertension (high blood pressure), tachycardia (fast heartbeat), hypokalemia (low blood levels of potassium), shaking, etc., might occur in some patients, but these effects have rarely, if at all, been observed in studies.[7]
Interactions
Based on theoretical considerations, co-application of other beta-adrenoceptor agonists, potassium lowering drugs (e.g. corticosteroids, many diuretics, and theophylline), tricyclic antidepressants, and monoamine oxidase inhibitors could increase the likelihood of adverse effects to occur. Beta blockers, a group of drugs for the treatment of hypertension (high blood pressure) and various conditions of the heart, could reduce the efficacy of olodaterol.[7] Clinical data on the relevance of such interactions are very limited.
Pharmacology
Mechanism of action
Like all β adrenoreceptor agonists, olodaterol mimics the effect of epinephrine at β2 receptors in the lung, which causes the bronchi to relax and reduces their resistance to airflow.[8]
Olodaterol is a nearly full β2 agonist, having 88% intrinsic activity compared to the gold standard isoprenaline/isoproterenol). Its half maximal effective concentration (EC50) is 0.1 nM. It has a higher in vitro selectivity for β2 receptors than the related drugs formoterol and salmeterol: 241-fold versus β1 and 2299-fold versus β3 adrenergic receptors.[9] The high β2/β1 selectivity may account for the apparent lack of tachycardia in clinical trials, which is mediated by β1 receptors on the heart.
Pharmacokinetics
Olodaterol is substantially metabolized by glucuronidation (UGT2B7, UGT1A1, UGT1A9) and O-demethylation (CYP2C8, CYP2C9).[2]
Pharmadynamics
Once bound to a β2 receptor, an olodaterol molecule stays there for hours — its dissociation half-life is 17.8 hours —, which allows for once-a-day application of the drug[8] like with indacaterol. Other related compounds generally have a shorter duration of action and have to be applied twice daily (e.g. formoterol, salmeterol). Still others (e. g. salbutamol/аlbuterol, fenoterol) have to be applied three or four times a day for continuous action, which can also be an advantage for patients who need to apply β2 agonists only occasionally, for example in an asthma attack.[10]
History
On 29 January 2013 the U.S. Food and Drug Administration (FDA) Pulmonary-Allergy Drugs Advisory Committee (PADAC) recommended that the clinical data included in the new drug application (NDA) for olodaterol provide substantial evidence of safety and efficacy to support the approval of olodaterol as a once-daily maintenance bronchodilator treatment for airflow obstruction in patients with COPD.[11]
On 18 October 2013 approval of olodaterol in the first three European countries — the United Kingdom, Denmark and Iceland — was announced by the manufacturer.[12]
On July 31, 2014 the U.S. Food and Drug Administration approved Striverdi Respimat (olodaterol inhalation spray) to treat patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema that are experiencing airflow obstruction.[13]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 "Olodaterol Monograph for Professionals". Drugs.com. Archived from the original on 23 January 2021. Retrieved 7 November 2021.
- 1 2 "Striverdi Respimat (olodaterol) Inhalation Spray For Oral Inhalation. U.S. Full Prescribing Information" (PDF). Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT 06877 USA. Archived (PDF) from the original on 5 March 2016. Retrieved 23 February 2016.
- ↑ "Olodaterol (Striverdi Respimat) Use During Pregnancy". Drugs.com. Archived from the original on 5 December 2020. Retrieved 7 November 2021.
- ↑ BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 268. ISBN 978-0857114105.
- ↑ "Striverdi Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 15 July 2016. Retrieved 7 November 2021.
- 1 2 Melani, AS (October 2018). "Olodaterol for the treatment of chronic obstructive pulmonary disease: a narrative review". Expert Opinion on Pharmacotherapy. 19 (14): 1603–1611. doi:10.1080/14656566.2018.1518431. PMID 30311516. S2CID 52964136.
- 1 2 Striverdi UK Drug Information
- 1 2 Casarosa P, Kollak I, Kiechle T, Ostermann A, Schnapp A, Kiesling R, et al. (June 2011). "Functional and biochemical rationales for the 24-hour-long duration of action of olodaterol" (PDF). The Journal of Pharmacology and Experimental Therapeutics. 337 (3): 600–9. doi:10.1124/jpet.111.179259. PMID 21357659. S2CID 15863445. Archived from the original on 2021-10-25. Retrieved 2021-04-07.
- ↑ Bouyssou T, Casarosa P, Naline E, Pestel S, Konetzki I, Devillier P, Schnapp A (July 2010). "Pharmacological characterization of olodaterol, a novel inhaled beta2-adrenoceptor agonist exerting a 24-hour-long duration of action in preclinical models". The Journal of Pharmacology and Experimental Therapeutics. 334 (1): 53–62. doi:10.1124/jpet.110.167007. PMID 20371707. S2CID 7994712.
- ↑ Haberfeld, H, ed. (2009). Austria-Codex (in Deutsch) (2009/2010 ed.). Vienna: Österreichischer Apothekerverlag. ISBN 978-3-85200-196-8.
- ↑ Hollis A (31 January 2013). "Panel Overwhelmingly Supports Boehringer COPD Drug Striverdi". FDA News/Drug Industry Daily. Archived from the original on 25 October 2021. Retrieved 7 April 2021.
- ↑ "New once-daily Striverdi (olodaterol) Respimat gains approval in first EU countries". Boehringer-Ingelheim. 18 October 2013. Archived from the original on 10 April 2021. Retrieved 7 April 2021.
- ↑ "FDA approves Striverdi Respimat to treat chronic obstructive pulmonary disease". FDA News/Drug Industry Daily. 31 July 2014. Archived from the original on 12 January 2017. Retrieved 7 April 2021.
External links
- Striverdi package leaflet (UK) Archived 2018-09-20 at the Wayback Machine
| Identifiers: |
|
|---|