Flunisolide
 | |
 | |
| Names | |
|---|---|
| Trade names | Aerospan, Aerobid, Nasalide, Nasarel, others |
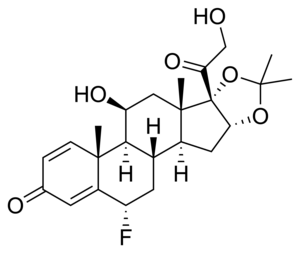
| Other names | 6α-Fluoro-11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione acetone cyclic 16,17-acetal |
IUPAC name
| |
| Clinical data | |
| Drug class | Corticosteroid[1][2] |
| Main uses | Allergic rhinitis, asthma, serous otitis media[1][2] |
| Side effects | Nose: Stinging, nose bleed, watery eyes, upper respiratory tract infection[1] Lungs: Throat inflammation, runny nose, headache, cough[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | Inhaled, in the nose |
| External links | |
| AHFS/Drugs.com | Inahled: Monograph Nose: Monograph |
| MedlinePlus | a681048 |
| Pharmacokinetics | |
| Protein binding | 40% after inhalation |
| Elimination half-life | 1.8 hours |
| Chemical and physical data | |
| Formula | C24H31FO6 |
| Molar mass | 434.504 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Flunisolide, sold under the brand name Aerospan among others, is a steroid used for allergic rhinitis, asthma, and serous otitis media.[2][1] Intranasal steroids are the most effective medication for controlling of allergic rhinitis.[3] For asthma it is inhaled.[2]
When used in the nose common side effects include stinging, nose bleed, watery eyes, and upper respiratory tract infection.[1] When inhaled common symptoms include throat inflammation, runny nose, headache, and cough.[2] Other side effects may include loss of smell and adrenal suppression.[1] It has strong glucocorticoid and weak mineralocorticoid effects and works by decreasing inflammation.[1][2]
Flunisolide was patented in 1958 and approved for medical use in 1978.[4] It is on the World Health Organization's List of Essential Medicines as an alternative to budesonide.[5] In the United States 25 ml of solution to spray in the nose costs about 26 USD, while 60 doses for inhaling costs about 102 USD as of 2021.[6][7]
Medical use
As-needed use has been shown to be not as effective as regular recommended use.[3] Flunisolide should not be used in the presence of nasal infection. It should not be continued if there is no relief of symptoms after regular use over two to three weeks. [8]
Side effects
Temporary nose and throat dryness, irritation, bleeding or unpleasant taste or smell may occur.[9] Nasal septum perforation is rarely reported.[3] Rare, but localized infections of the nose and pharynx with Candida albicans have been reported and long-term use may raise the chance of cataracts or glaucoma.[10]
Flunisolide nasal spray is absorbed into the circulatory system (blood).[8] Corticosteroid nasal sprays may affect the hypothalamic-pituitary-adrenal axis function in humans.[10] After the desired clinical effect is obtained, the maintenance dose should be reduced to the smallest amount necessary to control symptoms, which can be as low as 1 spray in each nostril a day. Utilizing the minimum effective dose will reduce possibility of side effects.[10] Recommended amounts of intranasal corticosteroids are generally not associated with systemic side effects.
Corticosteroids inhibit wound healing. Therefore, use of corticosteroid nasal sprays in patients who have experienced recent nasal septal ulcers, recurrent epistaxis, nasal surgery or trauma, a nasal corticosteroid should be used with caution until healing has occurred.[8] In pregnancy, recommended doses of intranasal corticosteroids are safe and effective.[3]
Mechanism of action
The principal mechanism of action of flunisolide is to activate glucocorticoid receptors, meaning it has an anti-inflammatory action. The effects of topical corticosteroids is not immediate and requires regular use and at least a few days to start experiencing noticeable symptom relief.
References
- 1 2 3 4 5 6 7 "Flunisolide (EENT) Monograph for Professionals". Drugs.com. Archived from the original on 7 April 2016. Retrieved 11 December 2021.
- 1 2 3 4 5 6 7 "Flunisolide Monograph for Professionals". Drugs.com. Archived from the original on 15 April 2017. Retrieved 11 December 2021.
- 1 2 3 4 Wallace DV, Dykewicz MS, Bernstein DI, Blessing-Moore J, Cox L, Khan DA, et al. (August 2008). "The diagnosis and management of rhinitis: an updated practice parameter". The Journal of Allergy and Clinical Immunology. 122 (2 Suppl): S1-84. doi:10.1016/j.jaci.2008.06.003. PMID 18662584.
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 486. ISBN 9783527607495. Archived from the original on 2021-08-28. Retrieved 2020-10-19.
- ↑ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ↑ "Flunisolide Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 9 July 2020. Retrieved 12 December 2021.
- ↑ "Aerospan HFA Prices, Coupons & Patient Assistance Programs". Drugs.com. Retrieved 12 December 2021.
- 1 2 3 "Flunisolide Nasal Solution". DailyMed. U.S. National Library of Medicine. Archived from the original on 2014-11-02. Retrieved 2020-10-19.
- ↑ "FLUNISOLIDE - NASAL (Nasalide, Nasarel) side effects, medical uses, and drug interactions". MedicineNet. Archived from the original on 2017-07-07. Retrieved 2020-10-19.
- 1 2 3 "Nasalide (Flunisolide (Nasal Spray)) Drug Information: Clinical Pharmacology - Prescribing Information". RxList. Archived from the original on 2016-03-04. Retrieved 2020-10-19.
External links
| Identifiers: |
|---|