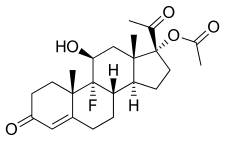
Flugestone acetate
 | |
| Clinical data | |
|---|---|
| Trade names | Cronolone, others |
| Other names | Flurogestone acetate; Fluorogestone acetate; FGA; NSC-65411; SC-9880; 17α-Acetoxy-9α-fluoro-11β-hydroxyprogesterone |
| Routes of administration | Intravaginal |
| Drug class | Progestogen; Progestin; Progestogen ester |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.017.979 |
| Chemical and physical data | |
| Formula | C23H31FO5 |
| Molar mass | 406.494 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Flugestone acetate (FGA), sold under the brand name Cronolone among others, is a progestin medication which is used in veterinary medicine.[1][2][3][4][5][6]
Uses
Veterinary
FGA is used as an intravaginal sponge preparation to synchronize estrus in ewes and goats.[2][4][5][6]
Chemistry
FGA, also known as 17α-acetoxy-9α-fluoro-11β-hydroxyprogesterone or as 17α-acetoxy-9α-fluoro-11β-hydroxypregn-4-ene-3,20-dione, is a synthetic pregnane steroid and a derivative of progesterone and 17α-hydroxyprogesterone.[1][3] It is the C17α acetate ester of flugestone.[1][3][2]
History
FGA was developed and marketed by G.D. Searle & Company in the 1960s.[7][8]
Society and culture
Generic names
Flugestone acetate is the generic name of the drug and its INN and BANM, while flurogestone acetate is its USAN.[1][3][2][9] Flugestone is the BAN and DCIT of the unesterified free alcohol form.[1][3][2][9] FGA is also known by its developmental code names NSC-65411 and SC-9880.[1][3][2][9]
Brand names
FGA is or has been marketed under the brand names Chronogest, Chrono-Gest, Crono-Gest, Cronolone, Gyncro-Mate, Ova-Gest, Ovakron, Synchro-Mate, Syncro Part, and Syncropart.[1][3][2][9]
Availability
FGA is marketed for veterinary use in Australia, France, Ireland, Israel, Italy, the Netherlands, South Africa, and the United Kingdom.[3][9]
References
- 1 2 3 4 5 6 7 Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. p. 559. ISBN 978-1-4757-2085-3.
- 1 2 3 4 5 6 7 Morton IK, Hall JM (31 October 1999). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 122–. ISBN 978-0-7514-0499-9.
- 1 2 3 4 5 6 7 8 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 451–. ISBN 978-3-88763-075-1.
- 1 2 Office of the Federal Register (US) (29 May 2012). Code of Federal Regulations Title 21: Food and Drug Administration: Parts 500-599, Revised April 1, 2012. Government Printing Office. pp. 359–. ISBN 978-0-16-090718-0.
- 1 2 Youngquist RS, Threlfall WR (23 November 2006). Current Therapy in Large Animal Theriogenology. Elsevier Health Sciences. pp. 2640–. ISBN 1-4377-1340-8.
- 1 2 Studdert VP, Gay CC, Blood DC (9 December 2011). Saunders Comprehensive Veterinary Dictionary. Elsevier Health Sciences. pp. 2673–. ISBN 978-0-7020-4744-2.
- ↑ Animal Husbandry Research: Reports on Agricultural Industry. 1967. p. 60.
- ↑ Dairy Science Handbook. Agriservices Foundation. 1971. p. 10.
- 1 2 3 4 5 "Flugestone". Drugs.com.