Selective progesterone receptor modulator
| Selective progesterone receptor modulator | |
|---|---|
| Drug class | |
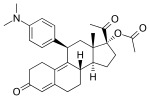 Ulipristal acetate, an SPRM that is used as an emergency contraceptive and in the treatment of uterine fibroids. | |
| Class identifiers | |
| Synonyms | SPRM |
| Use | Emergency contraception, uterine fibroids |
| ATC code | G03XB |
| Biological target | Progesterone receptor |
| Chemical class | Steroidal |
| In Wikidata | |
A selective progesterone receptor modulator (SPRM) is an agent that acts on the progesterone receptor (PR), the biological target of progestogens like progesterone. A characteristic that distinguishes such substances from full receptor agonists (e.g., progesterone, progestins) and full antagonists (e.g., aglepristone) is that their action differs in different tissues, i.e. agonist in some tissues while antagonist in others. This mixed profile of action leads to stimulation or inhibition in tissue-specific manner, which further raises the possibility of dissociating undesirable adverse effects from the development of synthetic PR-modulator drug candidates.[1]
History
Ever since the discovery of the progesterone hormone in the mid-1930s.[2][3] and especially after the discovery of its receptor in 1970[4][5] there has been a significant interest in developing an antagonistic agent for therapeutic use. Various progesterone analogs, known as progestins, were synthesized and in 1981 the first progesterone receptor antagonist was introduced by the name RU 38486 (RU 486, mifepristone).[6][7] However, the clinical limitation of mifepristone due to its relatively high binding affinity for glucocorticoid receptor compared to the progesterone receptor has sparked the demand for more selective progesterone antagonist to minimize risk of adverse effects.[7][8][9] As a contribution, so-called Selective Progesterone Receptor Modulators (SPRMs) have been developed. They have been described as agents with mixed antagonistic and agonistic effects on progesterone receptors in a tissue specific manner, while minimizing interactions with other steroidal receptors.[10][11] Opposed to progesterone antagonists, the mixed agonist-antagonist SPRM, due to their intrinsic progesterone agonistic activity, have an absent or only a minimal effect on pregnancy termination and are thus ideal for treating gynecological conditions without eliminating the potential of pregnancy.[12] Both steroidal[13] and non steroidal SPRMs[14] have been described and the most notable examples are asoprisnil,[15] which failed phase 3 clinical trial in 2008,[16] and ulipristal acetate,[17] the first SPRM on the market (2009 in Europe[18]).
Progesterone receptor
Receptor
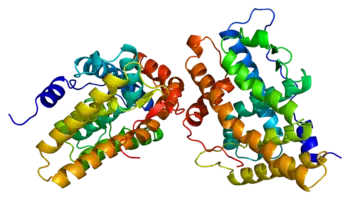
As a protein, the progesterone receptor (Fig. 1) is a member of the ligand-dependent nuclear hormone receptor family.[19] Two major progesterone receptor isoforms, A and B, as well as some other less common splice variants have been identified and they are all encoded by the same 8 exons gene.[20][21][22][23] Like other steroid nuclear receptors, the full-length protein, isoform B, can be divided into 4 functional regions, namely a variable N-terminal region followed by a highly conserved DNA-binding domain, variable hinge region and moderately conserved ligand binding domain.[20][21] The ligand binding site, known as AF2 domain , is expressed by exons 4-8, corresponding to 253 amino acids, and its structure is of great interest to SPRM development.[24] It consists of 10 α-helices (H1, H3-H12) forming 3 layered bundle entwined with 4 β-sheets . H12 is a condensed contiguous unit composed of helices 10 and 11, which has been suggested to participate in the process of co-activator binding.[25] The ligand binding domain of the receptor is in equilibrium between two different conformations. The first is an agonist conformation which favors the binding of coactivator proteins which in turn favors upregulation of gene transcription.[25] The second is an antagonistic conformation which in contrast favors the binding of corepressors and as a consequence down regulation of gene expression. Full agonists such as progesterone, which display agonist properties in all tissues, strongly shift the conformational equilibrium in the agonist direction.[25] Conversely full antagonists such as aglepristone strongly shift the equilibrium in the antagonist direction. Finally, the overall ratio of concentrations of coactivator to corepressor may differ in different cell types.[25]
G protein-coupled receptor
At the turn of the millennium it was apparent that progesterone activity was not mediated only via transcription factor, but also by a membrane-bound G protein-coupled receptor designated as 7TMPR. When the receptor is activated it blocks adenylyl cyclase, leading to decreased biosynthesis of the intracellular second-messenger cAMP.[24]
Downstream mechanisms
Since the 1990s it has been evident that the two major receptor isomers, A and B, are functionally distinct within the female reproductive system. Researches aimed at expression profile of the isomers suggests that the isomers are expressed in different tissues at different times throughout the menstrual cycle.[12] The PR-B has been found to be upregulated in the stroma and glandular epithelium during follicular phase, but is down-regluated in both tissues during luteal phase. On the contrary, PR-A is upregulated in both tissue types in the follicular phase and persists in the stromal tissue during the late luteal phase.[12] Studies have shown that PR-B activation is important for growth and development of the mammary gland, whereas PR-A has a significant role in normal reproductive function and ovulation. As well, in vitro researches have demonstrated that under identical conditions, the PR-B works as stronger transactivator of reporter genes, while PR-A is able to transrepress PR-B and other steroid receptors.[24] Various reasons have been found for this variety of function between the isoforms.[26] First to mention is that progesterone receptor isoform A lacks 164 N-terminal amino acids compared to isomer B, depriving it of the AF-3 activation function due to loss of B-upstream segment, which leaves it with only 2 activation functions.[27] Also, studies of mechanism have shown difference in cofactor recruitment between the isoforms. Due to these functional differences, one can see why there is an interest of developing a drug that can selectively target the receptor isoforms. Development of SPRMs has, in some cases, been focused on targeting these two different isoforms.[24][26][27]
SPRM interaction with receptor binding pockets
Certain interactions between ligand and progesterone receptor have been described to be important for ligand binding (Fig. 2). Crystallography studies of progesterone bound to its receptor have revealed an important hydrogen bond interaction between the progesterone electron-withdrawing 3-keto group and the residues Gln725 of helix-3 and Arg766 of helix-5, which are held in position by a structural water molecule.[26] This interaction has been shown to be present in interaction with various other ligands, e.g. mifepristone, tanaproget and asoprisnil and thus can be considered to be vital interaction for function of both agonists and antagonists.[28] Furthermore, progesterone and tanaproget, have been found to make a hydrogen bond with Asn719 in helix-3, giving an opportunity of higher selectivity and affinity, however, the SPRM asoprisnil has been found not to interact with this residue.[26] Even though the polar residue Thr894 is in close proximity to the C20 carbonyl group of progesterone there is not formed any hydrogen bond between these chemical groups. It is important to note the Thr894 has been found to interact with other ligands.[26][28]
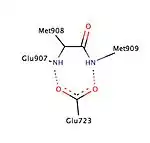
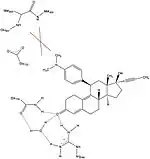
Various studies have described the presence of a hydrophobic pocket, referred as 17α pocket, which consists of Leu715, Leu718, Phe794, Leu797, Met801 and Tyr890 and appears to provide additional room for ligand expansion irrespective of agonism or antagonism. The 17α pocket, along with Met756 and Met759 within helix-5, as well as Met909, show a surprising flexibility in accommodation of various ligands, making the progesterone receptor very adaptive when it comes to binding.[26] Studies comparing the conformational changes in helix-12 contributing to agonistic and antagonistic effects have shown an important hydrogen interaction with Glu723 residue of helix-3. At inactive state the Glu723 stabilizes conformation of helix-12 by forming a hydrogen bond to main chain amines in Met908 and Met909.[26][28] When a ligand conducts an agonist effect, such as the oxime group of asoprisnil interacting with agonist binding pocket, then the hydrogen bond interaction between the previously mentioned residues in helix-12 and helix-3 strengthens, leading to docking and recruitment of coactivators. However, when an antagonist, e.g. mifepristone, interacts with this hydrogen bond system then its dimethylamine group clashes in to Met909 and destabilizes helix-12, causing a conformational change, which promotes the recruitment of corepressors.[26][28]
Mechanism of action
When SPRMs bind to the progesterone receptor, the equilibrium between the two conformational states is more closely balanced and hence more easily perturbed by differences in the cellular environment. In tissues where the concentration of coactivators is higher than corepressors, the excess coactivators drive the equilibrium in the agonist direction. Conversely in tissues where corepressor concentration is higher the equilibrium is driven in the antagonist direction.[29][30] Hence SPRMs display agonist activity in tissues where coactivators predominate and antagonist activity where corepressors are in excess.
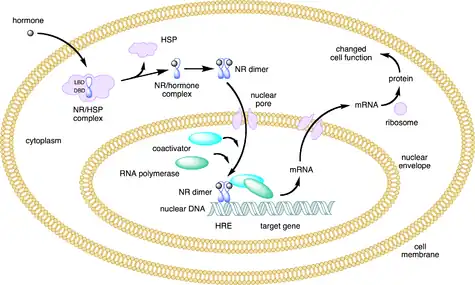
When inactive the progesterone receptor, as for other steroid receptor, forms a complex consisting of itself, heat shock proteins (hsp70, hsp90) and immunophilins.[31][32] Upon activation, due to hormone binding to ligand binding pocket, the receptor complex has been shown to dissociate, triggering nuclear import and giving the receptor the property of dimerisation (Fig. 3). In the nucleus the dimer interacts with progesterone hormone response element in the DNA causing upregulation or downregulation of the gene.[33][34][35][36] Various studies have demonstrated that it affects expression of up to 100 different genes, depending on receptor isomer.[26] In the action of agonism there occur conformational changes, where alpha helices 3, 4 and 12 create a docking surface for coactivator proteins, which act as bridging factors between the receptor and the general transcription machinery.[37][38] However, the antagonist prevents proper packing of alpha helix 12 against helices 3 and 4, impairing the receptor’s ability to interact with coactivators, which allows recruitment of corepressor, such as SMRT and NCoR.[39] Due to the minimal recruitment of corepressors during agonist binding then there has been postulated by Liu et al., 2002, that the ratio between coactivators vs. corepressors recruitment might be the determinant whether compound is considered to be an agonist, antagonist or mixed agonist-antagonist.[40] The selective progesterone receptor modulators have been described as agents with mixed agonist-antagonist activity and thus the mechanism of action must be due to a balance of these functions.
Structure-activity relationships
Steroidal SPRMs
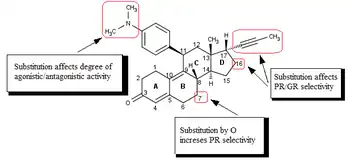
The research on mifepristone analogs, mainly focused on the improvement of the ratio of antiprogestational/antiglucocorticoid activity,[1][41] led to the discovery of SPRMs.[42] Modifications of or near the 17-alpha propinyl group (Fig. 4) on the D ring play a key role in binding to the progesterone receptor and/or glucocorticoid receptor.[41][42][43] Minor changes in the 17-alpha region generate antiprogestins with reduced antiglucocorticoidal activity, where alpha refers to an absolute steroidal stereodescriptor.[41][42][43][44][45][46] It seems that hydrophobic 17-alpha substituents such as 17-alpha ethyl and 17-alpha (1´-pentynyl) give rise to antiprogestational activity superior to that of mifepristone.[43] Substitution on the 17-alpha position involving phenyl group with small, electron-withdrawing substituents, such as F and CF3, on the para-position was also found to greatly increase the selectivity over glucocorticoid receptor as well as the potency of resulting compounds. Same substitution at the ortho- or meta- position led to decrease in selectivity. Bulky substituents, such as tert-butyl, in this region decrease the progesterone potency.[45]
The available biological and X-ray data suggest that the substitution of 4-(dimethylamino) phenyl group at the C11 (Fig. 4) position determines the degree of agonistic and antagonistic activity.[41][42] Small substituents like methyl or vinyl give rise to potent progesterone receptor-agonistic properties[42] whereas substituted phenyl derivatives show different degrees of antagonistic activity.[42][43][44] There is an indication, when substituted by various nitrogen heterocycles, that the most agonistic are compounds with a clear maximum in the negative electric potential in the region of the meta- and para- atoms of the aryl ring[47] whereas compounds that lack a center of electronegativity in this region have the highest antagonistic activity.[26][47]
Modification of the core steroidal structure affects the mode of binding to the progesterone receptor.[45][48] The substitution of C7 (Fig. 4) by oxygen atom has been investigated and these mifepristone-like oxasteroids showed increased selectivity over glucocorticoid receptor but were less potent than mifepristone.[45][49]
Nonsteroidal SPRMs
Progesterone receptor modulators with unique nonsteroidal structures are currently in the early stages of development (Fig. 5-12). Variety of new types of progesterone receptor antagonists with different degree of potency has been reported and show a remarkable structural diversity which can be seen in table below. Various lead compounds have also been identified as new progesterone receptor agonists. They can also be viewed in the table.[26]
| Antagonists | 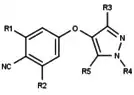 Figure 5: Progesterone receptor antagonists based on a pyrazole core | 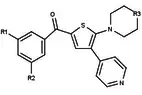 Figure 6: Trisubstituted thiophenes as PR antagonists with low potency | 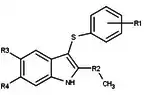 Figure 7: Indole derivatives as PR antagonists with preference of electron withdrawing groups on the aromatic ring | 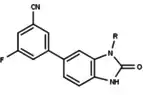 Figure 8: 6-aryl-1,3dihydrobenzimidazol -2ones substituent at the 1-position of the benzimidazolone |  Figure 9: PR antagonists with an aryl group inked to benzoxazin-2-one core through an amino group at the 6-position |
|---|---|---|---|---|---|
| Agonists | _benzoxazine-_2-thiones%252C_a_class_of_potent_and_selective_PR_agonists.png.webp) Figure 10: 6-(5-cyanopyrrol-2-yl) benzoxazine- 2-thiones | 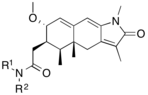 Figure 11: Tetrahydrobenzindolone lead compound with high selectivity | 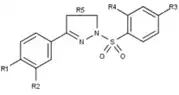 Figure 12: Arylpyrazolines and aryldiazepines as PR modulators |
Drugs
Members include:
- Ulipristal acetate ("Ella")
- Asoprisnil (J867; status uncertain)
- Telapristone (CDB-4124; Proellex, Progenta; under development)
SPRM have been suggested for multiple gynaecological applications, such as contraception and emergency contraception, treatment for endometriosis, uterine leiomyoma and as a hormone replacing therapy in post-menopausal women.[50] SPRM activity is mainly mediated via the progesterone receptor, where the endometrium is the major target tissue. In contrast to conventional progesterone antagonists, the SPRMs eliminate the ability to terminate pregnancy due to their mixed antagonist/agonist profile. Since SPRMs have a low affinity for the estrogen receptor, they are not thought to induce post-menopausal associated bone loss.[12] SPRMs use has been associated with endometrial metaplasia , which calls for the need for a long-term safety assessment.[12]
| Compound | Chemical structure |
|---|---|
| Ulipristal acetate |  Figure 13: Ulipristal acetate skeletal |
| Asoprisnil |  Asoprisnil |
| Telapristone |  Telapristone |
Ulipristal acetate
Ulipristal acetate (also known as CDB-2914)[51] (Fig. 13) is an 11-β aryl substituted SPRM that has been available as an emergency contraception in Europe since 2009 and was FDA approved in 2010.[52] It’s also marketed as a treatment for uterine leiomyoma in North America and Europe. As an emergency contraception ulipristal acetate has shown to be potent up to 120h after unprotected intercourse, compared to 72h potency of current emergency contraceptions.[50] In post-menopausal endometrium the compound seems to have antagonistic effect or progesterone receptor, indicating potential use in menopausal treatment but this has yet to be confirmed.[12]
Asoprisnil
Asoprisnil (J867) is a steroidal 11β-benzaldoxime substituted SPRM (Fig. 14).[15] The geometry of its oxime group is suggested to play a major role in the in vitro potency.[26] It has been suggested as a treatment for leiomyoma and endometriosis[53] and it is the first SPRM in the clinical development of endometriosis treatment to reach an advanced phase.[54]
Telapristone
Telapristone (CDB-4124), also known as Proellex (Fig. 15), entered phase II clinical trial for in treatment uterine fibroids in 2014[55] and has a planned phase II clinical trial for alleviation of symptoms of endometriosis in early 2016.[56][57] It has also been suggested to have chemopreventive effects.[58]
Uses
SPRMs are under development for the following uses:
- Asoprisnil and telapristone are both under investigation (2005) for the medical treatment of uterine leiomyoma.[59][60]
- Proellex has completed a number of clinical trials to treat endometriosis and uterine fibroids.[61]
While these SPRMs have been effective for the treatment of uterine fibroids, development of side effects such as endometrial thickening has limited their administration to no longer than three to four months.[60]
Future
Due to its antiglucocorticoidal activity, mifepristone is investigated for its therapeutical potential in indications like Cushing's syndrome, Alzheimer's disease or psychosis. Beside that SPRMs are under development for various gynecological applications, including estrogen-free contraception, uterine leiomyoma and endometriosis.[62]
See also
References
- 1 2 Chwalisz K, Perez MC, Demanno D, Winkel C, Schubert G, Elger W (May 2005). "Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis". Endocrine Reviews. 26 (3): 423–38. doi:10.1210/er.2005-0001. PMID 15857972.
- ↑ Misrahi M, Loosfelt H, Atger M, Mériel C, Zerah V, Dessen P, Milgrom E (Jun 1988). "Organisation of the entire rabbit progesterone receptor mRNA and of the promoter and 5' flanking region of the gene". Nucleic Acids Research. 16 (12): 5459–72. doi:10.1093/nar/16.12.5459. PMC 336778. PMID 3387238.
- ↑ Allen WM (Aug 1935). "The isolation of crystalline progestin". Science. 82 (2118): 89–93. Bibcode:1935Sci....82...89A. doi:10.1126/science.82.2118.89. PMID 17747122.
- ↑ Karrer P, Schwarzenbach G (Jan 1934). "Nachtrag betreffend Acidität und Reduktions-Vermögen der Ascorbinsäure". Helvetica Chimica Acta. 17 (1): 58–59. doi:10.1002/hlca.19340170111. ISSN 1522-2675.
- ↑ Sherman MR, Corvol PL, O'Malley BW (Nov 1970). "Progesterone-binding components of chick oviduct. I. Preliminary characterization of cytoplasmic components". The Journal of Biological Chemistry. 245 (22): 6085–96. doi:10.1016/S0021-9258(18)62667-5. PMID 5484467.
- ↑ Philibert D, Deraedt R, Deutsch G (1981). RU 38486: a potent antiglucocorticoid in vivo. The VII International Congress of Pharmacology. Japan: Tokyo.
- 1 2 Groyer A, Le Bouc Y, Joab I, Radanyi C, Renoir JM, Robel P, Baulieu EE (Jun 1985). "Chick oviduct glucocorticosteroid receptor. Specific binding of the synthetic steroid RU 486 and immunological studies with antibodies to chick oviduct progesterone receptor". European Journal of Biochemistry. 149 (2): 445–51. doi:10.1111/j.1432-1033.1985.tb08945.x. PMID 3996417.
- ↑ Gass EK, Leonhardt SA, Nordeen SK, Edwards DP (Apr 1998). "The antagonists RU486 and ZK98299 stimulate progesterone receptor binding to deoxyribonucleic acid in vitro and in vivo, but have distinct effects on receptor conformation". Endocrinology. 139 (4): 1905–19. doi:10.1210/endo.139.4.5944. PMID 9528977.
- ↑ Lázár G, Lázár G, Husztik E, Duda E, Agarwal MK (Jun 1995). "The influence of antiglucocorticoids on stress and shock". Annals of the New York Academy of Sciences. 761 (1): 276–95. Bibcode:1995NYASA.761..276L. doi:10.1111/j.1749-6632.1995.tb31384.x. PMID 7625726. S2CID 40422188.
- ↑ Spitz IM, Chwalisz K (Aug 2010). "Progesterone receptor modulators and progesterone antagonists in women's health". Steroids. 65 (10–11): 807–15. doi:10.1016/S0039-128X(00)00194-X. PMID 11108892. S2CID 27699000.
- ↑ Chen W, Ohara N, Wang J, Xu Q, Liu J, Morikawa A, Sasaki H, Yoshida S, Demanno DA, Chwalisz K, Maruo T (Apr 2006). "A novel selective progesterone receptor modulator asoprisnil (J867) inhibits proliferation and induces apoptosis in cultured human uterine leiomyoma cells in the absence of comparable effects on myometrial cells". The Journal of Clinical Endocrinology and Metabolism. 91 (4): 1296–304. doi:10.1210/jc.2005-2379. PMID 16464945.
- 1 2 3 4 5 6 Chabbert-Buffet N, Meduri G, Bouchard P, Spitz IM (2005). "Selective progesterone receptor modulators and progesterone antagonists: mechanisms of action and clinical applications". Human Reproduction Update. 11 (3): 293–307. doi:10.1093/humupd/dmi002. PMID 15790602.
- ↑ Elger W, Bartley J, Schneider B, Kaufmann G, Schubert G, Chwalisz K (2000-10-01). "Endocrine pharmacological characterization of progesterone antagonists and progesterone receptor modulators with respect to PR-agonistic and antagonistic activity". Steroids. 65 (10–11): 713–23. doi:10.1016/S0039-128X(00)00178-1. PMID 11108882. S2CID 46138800.
- ↑ Palmer S, Campen CA, Allan GF, Rybczynski P, Haynes-Johnson D, Hutchins A, Kraft P, Kiddoe M, Lai M, Lombardi E, Pedersen P, Hodgen G, Combs DW (Dec 2000). "Nonsteroidal progesterone receptor ligands with unprecedented receptor selectivity". The Journal of Steroid Biochemistry and Molecular Biology. 75 (1): 33–42. doi:10.1016/S0960-0760(00)00134-5. PMID 11179906. S2CID 19167595.
- 1 2 DeManno D, Elger W, Garg R, Lee R, Schneider B, Hess-Stumpp H, Schubert G, Chwalisz K (Nov 2003). "Asoprisnil (J867): a selective progesterone receptor modulator for gynecological therapy". Steroids. 68 (10–13): 1019–32. doi:10.1016/j.steroids.2003.09.008. PMID 14667995. S2CID 23074350.
- ↑ "Safety of Treatment of Uterine Fibroids With Asoprisnil - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 2016-01-11.
- ↑ Donnez J, Vázquez F, Tomaszewski J, Nouri K, Bouchard P, Fauser BC, Barlow DH, Palacios S, Donnez O, Bestel E, Osterloh I, Loumaye E (Jun 2014). "Long-term treatment of uterine fibroids with ulipristal acetate". Fertility and Sterility. 101 (6): 1565–73.e1–18. doi:10.1016/j.fertnstert.2014.02.008. PMID 24630081.
- ↑ "Assessment Report for Ellaone" (PDF). EMA. Retrieved Nov 2009.
{{cite web}}: Check date values in:|access-date=(help) - ↑ Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM (Dec 1995). "The nuclear receptor superfamily: the second decade". Cell. 83 (6): 835–9. doi:10.1016/0092-8674(95)90199-X. PMC 6159888. PMID 8521507.
- 1 2 Conneely OM, Maxwell BL, Toft DO, Schrader WT, O'Malley BW (Dec 1987). "The A and B forms of the chicken progesterone receptor arise by alternate initiation of translation of a unique mRNA". Biochemical and Biophysical Research Communications. 149 (2): 493–501. doi:10.1016/0006-291X(87)90395-0. PMID 3426587.
- 1 2 Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P (May 1990). "Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B". The EMBO Journal. 9 (5): 1603–14. doi:10.1002/j.1460-2075.1990.tb08280.x. PMC 551856. PMID 2328727.
- ↑ Hirata S, Shoda T, Kato J, Hoshi K (Dec 2002). "Novel isoforms of the mRNA for human female sex steroid hormone receptors". The Journal of Steroid Biochemistry and Molecular Biology. 83 (1–5): 25–30. doi:10.1016/S0960-0760(02)00255-8. PMID 12650698. S2CID 23588169.
- ↑ Hirata S, Shoda T, Kato J, Hoshi K (Apr 2003). "Isoform/variant mRNAs for sex steroid hormone receptors in humans". Trends in Endocrinology and Metabolism. 14 (3): 124–9. doi:10.1016/S1043-2760(03)00028-6. PMID 12670738. S2CID 46220794.
- 1 2 3 4 Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J (May 2008). "Progesterone receptors: form and function in brain". Frontiers in Neuroendocrinology. 29 (2): 313–39. doi:10.1016/j.yfrne.2008.02.001. PMC 2398769. PMID 18374402.
- 1 2 3 4 Ellmann S, Sticht H, Thiel F, Beckmann MW, Strick R, Strissel PL (Aug 2009). "Estrogen and progesterone receptors: from molecular structures to clinical targets". Cellular and Molecular Life Sciences. 66 (15): 2405–26. doi:10.1007/s00018-009-0017-3. PMID 19333551. S2CID 19975774.
- 1 2 3 4 5 6 7 8 9 10 11 12 Winneker RC, Fensome A, Zhang P, Yudt MR, McComas CC, Unwalla RJ (Aug 2008). "A new generation of progesterone receptor modulators". Steroids. 73 (7): 689–701. doi:10.1016/j.steroids.2008.03.005. PMID 18472121. S2CID 25094160.
- 1 2 Edwards DP, Altmann M, DeMarzo A, Zhang Y, Weigel NL, Beck CA (Jun 1995). "Progesterone receptor and the mechanism of action of progesterone antagonists". The Journal of Steroid Biochemistry and Molecular Biology. 53 (1–6): 449–58. doi:10.1016/0960-0760(95)00091-d. PMID 7626494. S2CID 41957428.
- 1 2 3 4 Lusher SJ, Raaijmakers HC, Vu-Pham D, Kazemier B, Bosch R, McGuire R, Azevedo R, Hamersma H, Dechering K, Oubrie A, van Duin M, de Vlieg J (Jun 2012). "X-ray structures of progesterone receptor ligand binding domain in its agonist state reveal differing mechanisms for mixed profiles of 11β-substituted steroids". The Journal of Biological Chemistry. 287 (24): 20333–43. doi:10.1074/jbc.M111.308403. PMC 3370215. PMID 22535964.
- ↑ Jackson TA, Richer JK, Bain DL, Takimoto GS, Tung L, Horwitz KB (Jun 1997). "The partial agonist activity of antagonist-occupied steroid receptors is controlled by a novel hinge domain-binding coactivator L7/SPA and the corepressors N-CoR or SMRT". Molecular Endocrinology. 11 (6): 693–705. doi:10.1210/me.11.6.693. PMID 9171233.
- ↑ Smith CL, O'Malley BW (Feb 2004). "Coregulator function: a key to understanding tissue specificity of selective receptor modulators". Endocrine Reviews. 25 (1): 45–71. doi:10.1210/er.2003-0023. PMID 14769827.
- ↑ Oñate SA, Estes PA, Welch WJ, Nordeen SK, Edwards DP (Dec 1991). "Evidence that heat shock protein-70 associated with progesterone receptors is not involved in receptor-DNA binding". Molecular Endocrinology. 5 (12): 1993–2004. doi:10.1210/mend-5-12-1993. PMID 1791844.
- ↑ Smith DF, Stensgard BA, Welch WJ, Toft DO (Jan 1992). "Assembly of progesterone receptor with heat shock proteins and receptor activation are ATP mediated events". The Journal of Biological Chemistry. 267 (2): 1350–6. doi:10.1016/S0021-9258(18)48438-4. PMID 1730655.
- ↑ DeMarzo AM, Beck CA, Onate SA, Edwards DP (Jan 1991). "Dimerization of mammalian progesterone receptors occurs in the absence of DNA and is related to the release of the 90-kDa heat shock protein". Proceedings of the National Academy of Sciences of the United States of America. 88 (1): 72–6. Bibcode:1991PNAS...88...72D. doi:10.1073/pnas.88.1.72. PMC 50750. PMID 1986383.
- ↑ Guiochon-Mantel A, Loosfelt H, Lescop P, Sar S, Atger M, Perrot-Applanat M, Milgrom E (Jun 1989). "Mechanisms of nuclear localization of the progesterone receptor: evidence for interaction between monomers". Cell. 57 (7): 1147–54. doi:10.1016/0092-8674(89)90052-4. PMID 2736623.
- ↑ O'Malley BW, Tsai MJ (Feb 1992). "Molecular pathways of steroid receptor action". Biology of Reproduction. 46 (2): 163–7. doi:10.1095/biolreprod46.2.163. PMID 1536890.
- ↑ Bagchi MK, Tsai MJ, O'Malley BW, Tsai SY (Aug 1992). "Analysis of the mechanism of steroid hormone receptor-dependent gene activation in cell-free systems". Endocrine Reviews. 13 (3): 525–35. doi:10.1210/edrv-13-3-525. PMID 1425487.
- ↑ McKenna NJ, O'Malley BW (Feb 2002). "Combinatorial control of gene expression by nuclear receptors and coregulators". Cell. 108 (4): 465–74. doi:10.1016/S0092-8674(02)00641-4. PMID 11909518.
- ↑ Oñate SA, Tsai SY, Tsai MJ, O'Malley BW (Nov 1995). "Sequence and characterization of a coactivator for the steroid hormone receptor superfamily". Science. 270 (5240): 1354–7. Bibcode:1995Sci...270.1354O. doi:10.1126/science.270.5240.1354. PMID 7481822. S2CID 28749162.
- ↑ Wagner BL, Norris JD, Knotts TA, Weigel NL, McDonnell DP (Mar 1998). "The nuclear corepressors NCoR and SMRT are key regulators of both ligand- and 8-bromo-cyclic AMP-dependent transcriptional activity of the human progesterone receptor". Molecular and Cellular Biology. 18 (3): 1369–78. doi:10.1128/mcb.18.3.1369. PMC 108850. PMID 9488452.
- ↑ Liu Z, Auboeuf D, Wong J, Chen JD, Tsai SY, Tsai MJ, O'Malley BW (Jun 2002). "Coactivator/corepressor ratios modulate PR-mediated transcription by the selective receptor modulator RU486". Proceedings of the National Academy of Sciences of the United States of America. 99 (12): 7940–4. Bibcode:2002PNAS...99.7940L. doi:10.1073/pnas.122225699. PMC 122999. PMID 12048256.
- 1 2 3 4 Nickisch K, Elger W, Cessac J, Kesavaram N, Das B, Garfield R, Shi SQ, Amelkina O, Meister R (Feb 2013). "Synthesis and biological evaluation of partially fluorinated antiprogestins and mesoprogestins". Steroids. 78 (2): 255–67. doi:10.1016/j.steroids.2012.09.010. PMID 23178161. S2CID 17158416.
- 1 2 3 4 5 6 Nickisch K, Elger W, Santhamma B, Garfield R, Killeen Z, Amelkina O, Schneider B, Meister R (Dec 2014). "Synthesis and biological evaluation of 11' imidazolyl antiprogestins and mesoprogestins". Steroids. 92: 45–55. doi:10.1016/j.steroids.2014.08.017. PMID 25174783. S2CID 6311225.
- 1 2 3 4 Rao PN, Wang Z, Cessac JW, Rosenberg RS, Jenkins DJ, Diamandis EP (Oct 1998). "New 11 beta-aryl-substituted steroids exhibit both progestational and antiprogestational activity". Steroids. 63 (10): 523–30. doi:10.1016/S0039-128X(98)00060-9. PMID 9800283. S2CID 42703467.
- 1 2 Wagner BL, Pollio G, Leonhardt S, Wani MC, Lee DY, Imhof MO, Edwards DP, Cook CE, McDonnell DP (Aug 1996). "16 alpha-substituted analogs of the antiprogestin RU486 induce a unique conformation in the human progesterone receptor resulting in mixed agonist activity". Proceedings of the National Academy of Sciences of the United States of America. 93 (16): 8739–44. Bibcode:1996PNAS...93.8739W. doi:10.1073/pnas.93.16.8739. PMC 38743. PMID 8710941.
- 1 2 3 4 Kang FA, Guan J, Jain N, Allan G, Linton O, Tannenbaum P, Chen X, Xu J, Zhu P, Gunnet J, Demarest K, Lundeen S, Sui Z (May 2007). "Parallel synthesis and SAR study of novel oxa-steroids as potent and selective progesterone receptor antagonists". Bioorganic & Medicinal Chemistry Letters. 17 (9): 2531–4. doi:10.1016/j.bmcl.2007.02.013. PMID 17317167.
- ↑ Kang FA, Allan G, Guan J, Jain N, Linton O, Tannenbaum P, Xu J, Zhu P, Gunnet J, Chen X, Demarest K, Lundeen S, Sui Z (Feb 2007). "Synthesis and identification of novel oxa-steroids as progesterone receptor antagonists". Bioorganic & Medicinal Chemistry Letters. 17 (4): 907–10. doi:10.1016/j.bmcl.2006.11.062. PMID 17169557.
- 1 2 Rewinkel J, Enthoven M, Golstein I, van der Rijst M, Scholten A, van Tilborg M, de Weys D, Wisse J, Hamersma H (Mar 2008). "11-(pyridinylphenyl)steroids--a new class of mixed-profile progesterone agonists/antagonists". Bioorganic & Medicinal Chemistry. 16 (6): 2753–63. doi:10.1016/j.bmc.2008.01.010. PMID 18243712.
- ↑ Jain N, Allan G, Linton O, Tannenbaum P, Chen X, Xu J, Zhu P, Gunnet J, Demarest K, Lundeen S, Murray W, Sui Z (Jul 2009). "Synthesis and SAR study of novel pseudo-steroids as potent and selective progesterone receptor antagonists". Bioorganic & Medicinal Chemistry Letters. 19 (14): 3977–80. doi:10.1016/j.bmcl.2009.01.095. PMID 19217285.
- ↑ Kang FA, Chen X, Jain N, Allan G, Tannenbaum P, Lundeen S, Sui Z (Jul 2008). "Insight from molecular modeling into different conformation and SAR of natural steroids and unnatural 7-oxa-steroids". Bioorganic & Medicinal Chemistry Letters. 18 (13): 3687–90. doi:10.1016/j.bmcl.2008.05.070. PMID 18539027.
- 1 2 Benagiano G, Bastianelli C, Farris M, Brosens I (Jul 2014). "Selective progesterone receptor modulators: an update". Expert Opinion on Pharmacotherapy. 15 (10): 1403–15. doi:10.1517/14656566.2014.914494. PMID 24787486. S2CID 31746942.
- ↑ Brache V, Cochon L, Jesam C, Maldonado R, Salvatierra AM, Levy DP, Gainer E, Croxatto HB (Sep 2010). "Immediate pre-ovulatory administration of 30 mg ulipristal acetate significantly delays follicular rupture". Human Reproduction. 25 (9): 2256–63. doi:10.1093/humrep/deq157. PMID 20634186.
- ↑ Aiken AR, Trussell J (2014). "Recent advances in contraception". F1000Prime Reports. 6: 113. doi:10.12703/p6-113. PMC 4251416. PMID 25580267.
- ↑ Spitz IM (Nov 2003). "Progesterone antagonists and progesterone receptor modulators: an overview". Steroids. 68 (10–13): 981–93. doi:10.1016/j.steroids.2003.08.007. PMID 14667991. S2CID 23054270.
- ↑ Lindsay SF, Luciano DE, Luciano AA (Sep 2015). "Emerging therapy for endometriosis". Expert Opinion on Emerging Drugs. 20 (3): 449–61. doi:10.1517/14728214.2015.1051966. PMID 26050551. S2CID 19276904.
- ↑ "A Phase 2, Study to Evaluate the Safety and Efficacy Proellex® (Telapristone Acetate) Administered Vaginally in the Treatment of Uterine Fibroids - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 2016-01-11.
- ↑ Eckstein N, Haas B, Hass MD, Pfeifer V (Aug 2014). "Systemic therapy of Cushing's syndrome". Orphanet Journal of Rare Diseases. 9 (1): 122. doi:10.1186/s13023-014-0122-8. PMC 4237936. PMID 25091295.
- ↑ Taylor DK, Holthouser K, Segars JH, Leppert PC (2015). "Recent scientific advances in leiomyoma (uterine fibroids) research facilitates better understanding and management". F1000Research. 4 (F1000 Faculty Rev): 183. doi:10.12688/f1000research.6189.1. PMC 4513689. PMID 26236472.
- ↑ Pabla B, Bissonnette M, Konda VJ (Oct 2015). "Colon cancer and the epidermal growth factor receptor: Current treatment paradigms, the importance of diet, and the role of chemoprevention". World Journal of Clinical Oncology. 6 (5): 133–41. doi:10.5306/wjco.v6.i5.133. PMC 4600187. PMID 26468449.
- ↑ Ohara N (2008). "Action of progesterone receptor modulators on uterine leiomyomas". Clinical and Experimental Obstetrics & Gynecology. 35 (3): 165–6. PMID 18754282.
- 1 2 Spitz IM (Aug 2009). "Clinical utility of progesterone receptor modulators and their effect on the endometrium". Current Opinion in Obstetrics and Gynecology. 21 (4): 318–24. doi:10.1097/GCO.0b013e32832e07e8. PMID 19602929. S2CID 2121292.
- ↑ "Proellex". ClinicalTrials.gov. U.S. National Institutes of Health. Retrieved 1 April 2021.
{{cite web}}: CS1 maint: url-status (link) - ↑ Bouchard P, Chabbert-Buffet N, Fauser BC (Nov 2011). "Selective progesterone receptor modulators in reproductive medicine: pharmacology, clinical efficacy and safety". Fertility and Sterility. 96 (5): 1175–89. doi:10.1016/j.fertnstert.2011.08.021. PMID 21944187.