Asoprisnil ecamate
 | |
| Clinical data | |
|---|---|
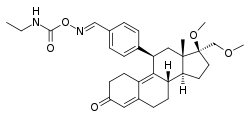
| Other names | J-956; 11β-(4-((E)-(Ethylcarbamoyl-oxyimino)methyl)phenyl)-17β-methoxy-17α-(methoxymethyl)estra-4,9-dien-3-one |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C31H40N2O5 |
| Molar mass | 520.670 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Asoprisnil ecamate (INN) (developmental code name J-956) is a synthetic, steroidal selective progesterone receptor modulator (SPRM) which was under development for the treatment of endometriosis, uterine fibroids, and menopausal symptoms but was discontinued.[1][2][3] It is a potent and highly selective ligand of the progesterone receptor with mixed agonistic and antagonistic activity and much reduced antiglucocorticoid activity relative to mifepristone.[2][3][4] The drug reached phase III clinical trials for the aforementioned indications prior to its discontinuation.[1]
See also
References
- 1 2 "Asoprisnil - AdisInsight".
- 1 2 Schubert G, Elger W, Kaufmann G, Schneider B, Reddersen G, Chwalisz K (2005). "Discovery, chemistry, and reproductive pharmacology of asoprisnil and related 11beta-benzaldoxime substituted selective progesterone receptor modulators (SPRMs)". Seminars in Reproductive Medicine. 23 (1): 58–73. doi:10.1055/s-2005-864034. PMID 15714390.
- 1 2 Chwalisz K, Perez MC, Demanno D, Winkel C, Schubert G, Elger W (2005). "Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis". Endocrine Reviews. 26 (3): 423–38. doi:10.1210/er.2005-0001. PMID 15857972.
- ↑ Chwalisz K, Garg R, Brenner R, Slayden O, Winkel C, Elger W (2006). "Role of nonhuman primate models in the discovery and clinical development of selective progesterone receptor modulators (SPRMs)". Reproductive Biology and Endocrinology. 4 Suppl 1: S8. doi:10.1186/1477-7827-4-S1-S8. PMC 1775068. PMID 17118172.
External links
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.