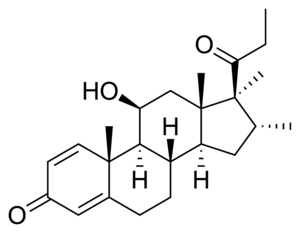
Rimexolone
 | |
| Names | |
|---|---|
| Trade names | Vexol |
| Other names | Trimexolone; Org 6216; 11β-Hydroxy-16α,17α,21-trimethylpregna-1,4-dien-3,20-dione |
IUPAC name
| |
| Clinical data | |
| Drug class | Corticosteroid |
| Main uses | Inflammation of the eye[1] |
| Side effects | Increased intraocular pressure, blurry vision, eye pain, redness, runny nose[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Eye drops |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a606003 |
| Pharmacokinetics | |
| Elimination half-life | estimated 1–2 hours |
| Excretion | >80% faeces |
| Chemical and physical data | |
| Formula | C24H34O3 |
| Molar mass | 370.533 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Rimexolone, sold under the brand name Vexol, is a steroid medication used to treat inflammation of the eye.[1] This includes anterior uveitis and following surgery of the eye.[1] It is used as an eye drop.[1]
Common side effects include increased intraocular pressure, blurry vision, eye pain, redness, and a runny nose.[1] Other complications can include fungal infection of the cornea.[1]
Rimexolone was approved for medical use in the United States in 1994.[1] In the United States a 5 ml bottle costs about 100 USD as of 2021.[2] It is no longer available in the United Kingdom as of 2019.[3]
Medical uses
Rimexolone is used to treat inflammation after eye surgery, to treat anterior uveitis, conjunctivitis and keratitis.[4][5]
Dosage
It is used as 1 to 2 drops four times per day.[1]
Contraindications
The substance is contraindicated in herpes simplex and most other viral eye infections, as well as mycobacterial, fungal and amoebal eye infections[4][5] because it only reduces the inflammation but does not act against such microorganisms.
Side effects
The most common adverse effects are blurred vision, tearing and other kinds of eye discomfort. Eye pain, eye oedema, headache, increased intraocular pressure and other side effects are seen in less than 1% of patients.[4][5]
Pharmacology
Pharmacodynamics
As a glucocorticoid, rimexolone acts as an agonist of the glucocorticoid receptor.
Pharmacokinetics
A small amount of rimexolone is absorbed into the systemic circulation. On hourly treatment with the eye drops for a week, blood serum concentrations peaked at 150 pg/ml on average, with many patients remaining below the detection threshold of 80 pg/ml. The elimination half-life from the circulation is estimated at one to two hours; the substance is mainly (over 80%) excreted via the faeces.[4][5]
References
- 1 2 3 4 5 6 7 8 9 "Rimexolone Monograph for Professionals". Drugs.com. Archived from the original on 20 January 2021. Retrieved 17 October 2021.
- ↑ "Vexol Prices, Coupons & Patient Assistance Programs". Drugs.com. Retrieved 17 October 2021.
- ↑ "Rimexolone eye drops. Rimexolone antibiotic eye drops - Patient". patient.info. Archived from the original on 13 August 2021. Retrieved 17 October 2021.
- 1 2 3 4 Haberfeld, H, ed. (2015). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag. Vexol 1% (10 mg/ml)-Augentropfensuspension.
{{cite book}}: CS1 maint: unrecognized language (link) - 1 2 3 4 Drugs.com: Monograph.
External links
| Identifiers: |
|---|