Resocortol
 | |
| Clinical data | |
|---|---|
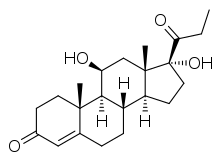
| Other names | 11β,17α-Dihydroxy-21-methylpregn-4-ene-3,20-dione |
| Drug class | Corticosteroid; Glucocorticoid |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C22H32O4 |
| Molar mass | 360.494 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Resocortol is a synthetic glucocorticoid corticosteroid which was never marketed.[1][2]
References
- ↑ Challener CA (1 December 2001). Chiral Drugs. Wiley. p. 311. ISBN 978-0-566-08411-9.
- ↑ Coert A, Verheijen F, Horspool LJ, Mol JA (October 2004). "Aspects of pharmacodynamics and biotransformation of the glucocorticoid resocortol butyrate". Journal of Veterinary Pharmacology and Therapeutics. 27 (5): 309–15. doi:10.1111/j.1365-2885.2004.00583.x. PMID 15500568.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.