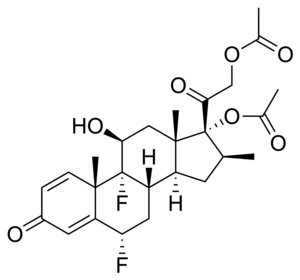
Diflorasone diacetate
 | |
| Names | |
|---|---|
| Trade names | Psorcon, Florone, others |
IUPAC name
| |
| Clinical data | |
| Drug class | Topical steroid[1] |
| Main uses | Atopic dermatitis, psoriasis, allergic contact dermatitis[1] |
| Side effects | Irritation, folliculitis, acne, decreased pigmentation, perioral dermatitis, infection, increased hair, striae[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Topical |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a602019 |
| Legal | |
| License data |
|
| Legal status |
|
| Chemical and physical data | |
| Formula | C26H32F2O7 |
| Molar mass | 494.532 g·mol−1 |
InChI
| |
Diflorasone diacetate, sold under the brand name Psorcon among others, is a topical steroid.[1] It is used for atopic dermatitis, psoriasis, and allergic contact dermatitis.[1]
Common side effects include irritation, folliculitis, acne, decreased pigmentation, perioral dermatitis, infection, increased hair, and striae.[1] Other side effects may include Cushing's syndrome and allergic reactions.[1] Safety in pregnancy is unclear.[1] The strength in the United States is classified as group III.[2]
Diflorasone diacetate was approved for medical use in the United States in 1977.[1] It is available as a generic medication.[3] In the United States a 30 gram tube costs about 65 USD as of 2021.[3]
Medical uses
Dosage
It may be used once to four times per day.[1]
Side effects
No long-term animal studies have been done to determine whether diflorasone diacetate could have carcinogenic properties.
Little data is available regarding whether diflorasone diacetate would be present in great enough quantities to cause harm to a infant.[4]
Society and culture
It is manufactured by E. Fougera & Co.
References
- 1 2 3 4 5 6 7 8 9 10 "Diflorasone Monograph for Professionals". Drugs.com. Archived from the original on 19 January 2021. Retrieved 28 December 2021.
- ↑ "Topical Corticosteroids: Overview". 9 July 2021. Archived from the original on 19 August 2021. Retrieved 28 December 2021.
- 1 2 "Diflorasone Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 12 May 2016. Retrieved 28 December 2021.
- ↑ "Diflorasone topical". Drugs.com. Archived from the original on 2020-11-17. Retrieved 2020-11-12.
External links
| Identifiers: |
|---|
- "Diflorasone diacetate". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2020-11-12. Retrieved 2020-11-12.