Triamcinolone
 | |
 | |
| Names | |
|---|---|
| Trade names | Kenalog, Nasacort, others |
| Other names |
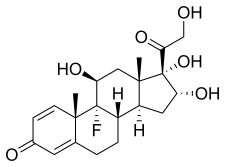
(8S,9R,10S,11S,13S,14S,16R,17S)-9-fluoro-11,16,17-trihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-3-one; (1R,2S,10S,11S,13R,14S,15S,17S)-1-fluoro-13,14,17-trihydroxy-14-(2-hydroxyacetyl)-2,15-dimethyltetracyclo[8.7.0.02,7.011,15]heptadeca-3,6-dien-5-one |
IUPAC name
| |
| Clinical data | |
| Main uses | Skin diseases, allergies, rheumatic disorders[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth, topical, IM, intra-articular, intrasynovial |
| Defined daily dose | 7.5 mg[2] |
| External links | |
| AHFS/Drugs.com | Topical: Monograph Systemic: Monograph ENT: Monograph |
| MedlinePlus | a601122 |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Protein binding | 68% |
| Metabolism | Liver |
| Elimination half-life | 88 minutes |
| Excretion | Fecal and Kidney |
| Chemical and physical data | |
| Formula | C21H27FO6 |
| Molar mass | 394.439 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Triamcinolone is a glucocorticoid used to treat certain skin diseases, allergies, and rheumatic disorders among others.[1] It is also used to prevent worsening of asthma and COPD.[1] It can be taken in various ways including by mouth, injection into a muscle, and inhalation.[1]
Common side effects with long term use include osteoporosis, cataracts, thrush, and muscle weakness.[1] Serious side effects may include psychosis, increased risk of infections, adrenal suppression, and bronchospasm.[1] Use in pregnancy is generally safe.[4] It works by decreasing inflammation and immune system activity.[1]
Triamcinolone was patented in 1956 and came into medical use in 1958.[5] It is on the World Health Organization's List of Essential Medicines as an alternative for triamcinolone hexacetonide.[6] It is available as a generic medication.[7] In the United States the wholesale cost is about US$0.06 per gram for the cream.[8] In 2017, it was the 103rd most commonly prescribed medication in the United States, with more than seven million prescriptions.[9][10]
Medical uses
Triamcinolone is used to treat a number of different medical conditions, such as eczema, lichen sclerosus, psoriasis, arthritis, allergies, ulcerative colitis, lupus, sympathetic ophthalmia, temporal arteritis, uveitis, ocular inflammation, keloids, urushiol-induced contact dermatitis, aphthous ulcers (usually as triamcinolone acetonide), central retinal vein occlusion, visualization during vitrectomy and the prevention of asthma attacks.[11][12][13]
The derivative triamcinolone acetonide is the active ingredient in various topical skin preparations (cream, lotion, ointment, aerosol spray) designed to treat skin conditions such as rash, inflammation, redness, or intense itching due to eczema[14] and dermatitis.[15]
.jpg.webp) Intralesional steroid injection
Intralesional steroid injection.jpg.webp) Intralesional steroid injection
Intralesional steroid injection
Dosage
The defined daily dose is 7.5 mg by mouth or by injection.[2]
Side effects
Side effects of triamcinolone include sore throat, nosebleeds, increased coughing, headache, and runny nose. White patches in the throat or nose indicate a serious side effect. Symptoms of an allergic reaction include rash, itch, swelling, severe dizziness, trouble breathing.[16]
Chemistry
Triamcinolone is a synthetic pregnane corticosteroid and derivative of cortisol (hydrocortisone) and is also known as 1-dehydro-9α-fluoro-16α-hydroxyhydrocortisone or 9α-fluoro-16α-hydroxyprednisolone as well as 9α-fluoro-11β,16α,17α,21-tetrahydroxypregna-1,4-diene-3,20-dione.[17][18]
Society and culture
In 2010, TEVA and Perrigo launched the first generic inhalable triamcinolone.[19]
According to Chang et al. (2014), "Triamcinolone acetonide (TA) is classified as an S9 glucocorticoid in the 2014 Prohibited List published by the World Anti-Doping Agency, which caused it to be prohibited in international athletic competition when administered orally, intravenously, intramuscularly or rectally".[20]
Cost
In the United States the wholesale cost is about US$0.06 per gram for the cream.[8] In 2017, it was the 103rd most commonly prescribed medication in the United States, with more than seven million prescriptions.[9][10]
.svg.png.webp) Triamcinolone costs (US)
Triamcinolone costs (US).svg.png.webp) Triamcinolone prescriptions (US)
Triamcinolone prescriptions (US)
Brand names
Trade names for triamcinolone include Aristocort (Sandoz, now Novartis), Azmacort (KOS), Kenacort (Bristol-Myers Squibb), Kenalog (Bristol-Myers Squibb), Nincort, Ratio-Triacomb, Triaderm (Schering-Plough), Trianex, Tricort (Cadila), Tricortone, Trilone, Tristoject, Volon A.
 Aristocort (triamcinolone) topical cream
Aristocort (triamcinolone) topical cream Kenalog (triamcinolone) IM injection
Kenalog (triamcinolone) IM injection
See also
- Glucocorticoid (a chart comparing various glucocorticoids)
- Triamcinolone acetonide
References
- 1 2 3 4 5 6 7 "Triamcinolone Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 7 August 2019. Retrieved 3 March 2019.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 1 July 2020. Retrieved 5 September 2020.
- ↑ British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 1172. ISBN 9780857113382.
- ↑ "Triamcinolone Use During Pregnancy". Drugs.com. Archived from the original on 24 January 2021. Retrieved 3 March 2019.
- ↑ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 486. ISBN 978-3-527-60749-5. Archived from the original on 2016-12-22. Retrieved 2019-03-01.
- ↑ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ↑ Vallerand, April Hazard (2018). Davis's Drug Guide for Nurses. F.A. Davis. p. 365. ISBN 978-0-8036-7000-6. Archived from the original on 2021-08-29. Retrieved 2019-03-09.
- 1 2 "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Archived from the original on 2019-03-06. Retrieved 3 March 2019.
- 1 2 "The Top 300 of 2020". ClinCalc. Archived from the original on 18 March 2020. Retrieved 11 April 2020.
- 1 2 "Triamcinolone - Drug Usage Statistics". ClinCalc. Archived from the original on 12 April 2020. Retrieved 11 April 2020.
- ↑ "Triamcinolone – Drugs.com". Archived from the original on 2017-07-16. Retrieved 2018-01-23.
- ↑ "Triamcinolone Inhalation – Drugs.com". Archived from the original on 2018-01-31. Retrieved 2018-01-23.
- ↑ "Alcon Receives FDA Approval of Triesence Injectable Triamcinolone Suspension for Use in Eye Surgery – Drugs.com". Archived from the original on 2017-02-02. Retrieved 2018-01-23.
- ↑ Chong M, Fonacier L (December 2016). "Treatment of Eczema: Corticosteroids and Beyond". Clinical Reviews in Allergy & Immunology. 51 (3): 249–262. doi:10.1007/s12016-015-8486-7. PMID 25869743.
- ↑ Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. (July 2014). "Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies". Journal of the American Academy of Dermatology. 71 (1): 116–32. doi:10.1016/j.jaad.2014.03.023. PMC 4326095. PMID 24813302.
Topical corticosteroids (TCS) are used in the management of AD in both adults and children and are the mainstay of anti-inflammatory therapy.
- ↑ "Drugs and Treatments – Nasacort AQ Nasl – Patient Handout". WebMD. Archived from the original on 2008-04-16. Retrieved 2008-03-24.
- ↑ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 1228–. ISBN 978-1-4757-2085-3. Archived from the original on 5 August 2017. Retrieved 15 December 2016.
- ↑ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 1054–. ISBN 978-3-88763-075-1. Archived from the original on 2021-08-29. Retrieved 2016-12-15.
- ↑ "Perrigo Announces Launch Of Generic Version Of Nasacort AQ – CBS Detroit". Archived from the original on 2011-11-07. Retrieved 2011-08-24.
- ↑ Chang CW, Huang TY, Tseng YC, Chang-Chien GP, Lin SF, Hsu MC (November 2014). "Positive doping results caused by the single-dose local injection of triamcinolone acetonide". Forensic Science International. 244: 1–6. doi:10.1016/j.forsciint.2014.07.024. PMID 25126738.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- "Triamcinolone Topical". MedlinePlus. Archived from the original on 2008-10-01. Retrieved 2020-05-15.
- "Triamcinolone Nasal Spray". MedlinePlus. Archived from the original on 2020-08-04. Retrieved 2020-05-15.