Ulobetasol propionate
 | |
| Clinical data | |
|---|---|
| Trade names | Ultravate, Lexette, Bryhali |
| Other names | Halobetasol propionate, halobetasol propionate (USAN US) |
| AHFS/Drugs.com | Professional Drug Facts |
| MedlinePlus | a601060 |
| Routes of administration | Topical |
| Drug class | Corticosteroid; Glucocorticoid |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.211.977 |
| Chemical and physical data | |
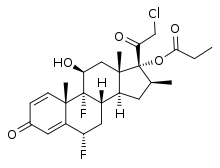
| Formula | C25H31ClF2O5 |
| Molar mass | 484.96 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Ulobetasol propionate, also known as halobetasol propionate and sold under the brand name Ultravate among others, is a synthetic glucocorticoid corticosteroid and a corticosteroid ester.
It was patented in 1975 and approved for medical use in 1990.[1][2]
References
- ↑ "Ultravate: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Retrieved 29 April 2020.
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 487. ISBN 9783527607495.
External links
- "Halobetasol propionate". Drug Information Portal. U.S. National Library of Medicine.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.