Fluocinolone
 | |
.png.webp) | |
| Names | |
|---|---|
| Trade names | Synalar, Iluvien, others |
| Other names | Fluocinolone acetonide |
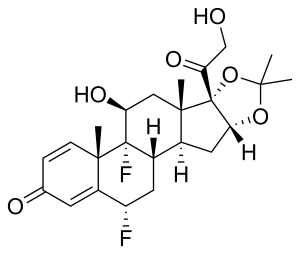
IUPAC name
| |
| Clinical data | |
| Drug class | Corticosteroid (glucocorticoid) |
| Main uses | Skin: Eczema, psoriasis[1] Eye: Uveitis, diabetic macular edema[2][1] |
| Side effects | Skin: Irritation, dry skin, folliculitis, acne, decreased pigmentation, skin atrophy, infection[3] Eye: Increased eye pressure, eye pain, conjunctival bleeding, blurry vision, dry eyes[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | Topical |
| External links | |
| AHFS/Drugs.com | Topical: Monograph Eyes: Monograph |
| Legal | |
| Legal status | |
| Pharmacokinetics | |
| Metabolism | Liver, CYP3A4-mediated |
| Elimination half-life | 1.3 to 1.7 hours |
| Chemical and physical data | |
| Formula | C24H30F2O6 |
| Molar mass | 452.495 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Fluocinolone, sold under the brand name Synalar among others, is a corticosteroid.[2] It is applied to the skin to treat eczema and psoriasis.[1] It is used as an implant within the eye to treat uveitis and vision problems due to diabetic macular edema.[2][1]
When applied to the skin common side effects include irritation, dry skin, folliculitis, acne, decreased pigmentation, skin atrophy, and infection.[3] When used in the eye common side effects include increased eye pressure, eye pain, conjunctival bleeding, blurry vision, and dry eyes.[2] Other complications may include cataracts, retinal detachment, and infection in the eye.[2] It works by decreasing inflammation.[3]
Fluocinolone was first made in 1959.[4] It was approved for medical use in 1961.[5] In the United Kingdom it costs the NHS about £10 for a tube of 60 grams and about £5,500 for a dose to place in the eye as of 2021.[1] This amount in the United States costs about 30 USD and 9,200 USD respectively.[6][7]
Medical uses
Fluocinolone acetonide intravitreal implants have been used to treat non-infectious uveitis. A systematic review could not determine whether fluocinolone acetonide implants are superior to standard of care treatment for uveitis.[8]
The cream can be mild to strong depending on the concentration.[1]
Dosage
It may be given by injection into the eye of 190 micrograms.[1]
Chemistry
It is used as fluocinolone acetonide, a salt of fluocinolone.[2] Chemically it is known as 6α-Fluorotriamcinolone; 6α,9α-Difluoro-11β,16α,17α,21-tetrahydroxypregna-1,4-diene-3,20-dione.
Classification
Fluocinolone is a group V (0.025%) or group VI (0.01%) corticosteroid under US classification.
Society and culture
A fluocinolone acetonide intravitreal implant with the brand name "Iluvien" is sold by biopharmaceutical company Alimera Sciences to treat diabetic macular edema (DME).[9]
References
- 1 2 3 4 5 6 7 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. pp. 1236, 1291. ISBN 978-0857114105.
- 1 2 3 4 5 6 7 "Fluocinolone (EENT) Monograph for Professionals". Drugs.com. Archived from the original on 27 November 2021. Retrieved 12 December 2021.
- 1 2 3 "Fluocinolone (Topical) Monograph for Professionals". Drugs.com. Archived from the original on 27 November 2021. Retrieved 14 December 2021.
- ↑ J S Mills, A. Bowers, Carl Djerassi and H.J. Ringold, Steroids CXXXVII. Synthesis of a New Class of Potent Cortical Hormones. 6α,9α-Difluoro-16α-Hydroxyprednisolone and its Acetonide, Journal of the American Chemical Society, 80, 3399-3404 (1960)
- ↑ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 485. ISBN 9783527607495. Archived from the original on 2020-08-01. Retrieved 2021-12-12.
- ↑ "Fluocinolone Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 14 December 2021.
- ↑ "Iluvien Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 21 April 2021. Retrieved 14 December 2021.
- ↑ Brady CJ, Villanti AC, Law HA, Rahimy E, Reddy R, Sieving PC, Garg SJ, Tang J (2016). "Corticosteroid implants for chronic non-infectious uveitis". Cochrane Database Syst Rev. 2: CD010469. doi:10.1002/14651858.CD010469.pub2. PMC 5038923. PMID 26866343.
- ↑ "Real-world study shows long-term safety, efficacy of Iluvien in DME". Healio. 2020-07-02. Archived from the original on 2020-08-15. Retrieved 2020-10-28.
External links
- "Fluocinolone acetonide". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-12-07. Retrieved 2021-12-12.
| Identifiers: |
|---|