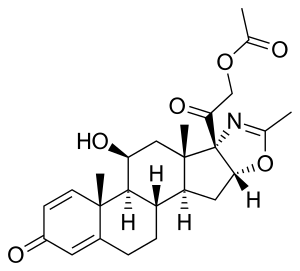
Deflazacort
 | |
| Names | |
|---|---|
| Trade names | Calcort, Emflaza, others |
IUPAC name
| |
| Clinical data | |
| Drug class | Glucocorticoid[1] |
| Main uses | Allergic disorders, inflammatory disorders[1] |
| Side effects | Weight gain, upper respiratory tract infection, increased hair growth, red skin, irritability[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| Legal status | |
| Pharmacokinetics | |
| Protein binding | 40% |
| Metabolism | By plasma esterases, to active metabolite |
| Elimination half-life | 1.1–1.9 hours (metabolite) |
| Excretion | Kidney (70%) and fecal (30%) |
| Chemical and physical data | |
| Formula | C25H31NO6 |
| Molar mass | 441.524 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Deflazacort, sold under the trade name Calcort among others, is a glucocorticoid used to treat allergic disorders and inflammatory disorders.[1] It may also be used for Duchenne muscular dystrophy.[2] It is taken by mouth.[1]
Common side effects include weight gain, upper respiratory tract infection, increased hair growth, red skin, and irritability.[2] Other side effects may include infection, adrenal insufficiency, Cushing syndrome, high blood sugar, anaphylaxis, blood clots, and toxic epidermal necrolysis.[2] Deflazacort 6 mg has a similar anti-inflammatory effect as prednisone 5 mg.[1]
Deflazacort was patented in 1965 and approved for medical use in 1985.[3] In the United Kingdom 60 tablets of 6 mg costs the NHS about £16 as of 2021.[1] In the United States this amount costs about 4,100 USD.[4]
Medical uses
The manufacturer lists the following uses for deflazacort:[5]
- Acute interstitial nephritis
- Anaphylaxis
- Asthma
- Autoimmune haemolytic anaemia
- Bullous pemphigoid
- Mixed connective tissue disease (other than systemic sclerosis)
- Crohn's disease
- Dermatomyositis
- Idiopathic thrombocytopenic purpura
- Juvenile chronic arthritis
- Severe hypersensitivity reactions
- Immunosuppression in transplantation
- Acute and lymphatic leukaemia
- Malignant lymphoma
- Multiple myeloma
- Muscular dystrophy
- Rheumatoid arthritis
- Polymyalgia rheumatica
- Nephrotic syndrome
- Pemphigus
- Polyarteritis nodosa
- Pyoderma gangrenosum
- Sarcoidosis
- Systemic lupus erythematosus
- Ulcerative colitis
In the United States, deflazacort is approved for the treatment of duchenne muscular dystrophy in people over the age of five.[6]
Side effects
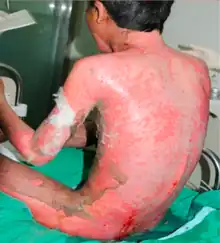
Deflazacort carries the risks common to all corticosteroids, including immune suppression, decreased bone density, and endocrine insufficiency. In clinical trials, the most common side effects (>10% above placebo) were Cushing's-like appearance, weight gain, and increased appetite.[7]
Pharmacology
Mechanism of action
Deflazacort is an inactive prodrug which is metabolized rapidly to the active drug 21-desacetyldeflazacort.[8]
Relative potency
Deflazacort's potency is around 70–90% that of prednisone.[9] A 2017 review found its activity of 7.5 mg of deflazacort is approximately equivalent to 25 mg cortisone, 20 mg hydrocortisone, 5 mg of prednisolone or prednisone, 4 mg of methylprednisolone or triamcinolone, or 0.75 mg of betamethasone or dexamethasone. The review noted that the drug has a high therapeutic index, being used at initial oral doses ranging from 6 to 90 mg, and probably requires a 50% higher dose to induce the same demineralizing effect as prednisolone. Thus it has "a smaller impact on calcium metabolism than any other synthetic corticosteroid, and therefore shows a lower risk of growth rate retardation in children and of osteoporosis" in the elderly, and comparatively small effects on carbohydrate metabolism, sodium retention, and hypokalemia.[10]
History
In January 2015, the FDA granted fast track status to Marathon Pharmaceuticals to pursue approval of deflazacort as a potential treatment for Duchenne muscular dystrophy, a rare, "progressive and fatal disease" that affects boys.[11] Although deflazacort was approved by the FDA for use in treatment of Duchenne muscular dystrophy on February 9, 2017,[12][13] Marathon CEO announced on February 13, 2017 that the launch of deflazacort (Emflaza) would be delayed amidst controversy over the steep price Marathon was asking for the drug in the United States - $89,000 per year, which is "roughly 70 times" more than it would cost overseas.[14] Because deflazacort is an older drug which has been long-approved in some other countries, it is now available in many places as an inexpensive generic. For example, in Canada deflazacort can be purchased for around $1 per tablet.[15]
Deflazacort is sold in the United States under the brand name Emflaza after PTC Therapeutics, Inc. acquired all rights to Emflaza on March 16, 2017.[16] Deflazacort is sold in the United Kingdom under the trade name Calcort;[9] in Brazil as Cortax, Decortil, Defcort and Deflanil; in India as Moaid, Zenflav, Defolet, DFZ, Decotaz, and DefZot; in Bangladesh as Xalcort; in Panama as Zamen; Spain as Zamene; and in Honduras as Flezacor.[17]
The U.S. Food and Drug Administration approved deflazacort to treat people age five years and older with Duchenne muscular dystrophy (DMD), a rare genetic disorder that causes progressive muscle deterioration and weakness. Emflaza is a corticosteroid that works by decreasing inflammation and reducing the activity of the immune system.[6] NDA 208684 was approved on February 9, 2017 as a Type 1- new molecular entity with orphan status.[18]
The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication for Duchenne muscular dystrophy.[19]
References
- 1 2 3 4 5 6 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 714. ISBN 978-0857114105.
- 1 2 3 4 "Deflazacort Monograph for Professionals". Drugs.com. Archived from the original on 12 August 2020. Retrieved 22 December 2021.
- ↑ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 486. ISBN 9783527607495. Archived from the original on 2021-08-28. Retrieved 2021-07-01.
- ↑ "Emflaza Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 14 August 2020. Retrieved 22 December 2021.
- ↑ "Refla: deflazacort" (PDF). Archived (PDF) from the original on 2017-02-11. Retrieved 2021-07-01.
- 1 2 "FDA approves drug to treat Duchenne muscular dystrophy". U.S. Food and Drug Administration (Press release). Archived from the original on 30 May 2019. Retrieved 18 February 2017.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Emflaza- deflazacort tablet Emflaza- deflazacort suspension". DailyMed. 29 January 2020. Archived from the original on 29 September 2020. Retrieved 17 September 2020.
- ↑ Möllmann H, Hochhaus G, Rohatagi S, Barth J, Derendorf H (July 1995). "Pharmacokinetic/pharmacodynamic evaluation of deflazacort in comparison to methylprednisolone and prednisolone". Pharmaceutical Research. 12 (7): 1096–100. doi:10.1023/a:1016287104656. PMID 7494809. S2CID 9920545.
- 1 2 "Calcort". electronic Medicines Compendium. June 11, 2008.
{{cite web}}: CS1 maint: url-status (link) Retrieved on October 28, 2008. - ↑ Parente L (January 2017). "Deflazacort: therapeutic index, relative potency and equivalent doses versus other corticosteroids". BMC Pharmacology & Toxicology. 18 (1): 1. doi:10.1186/s40360-016-0111-8. PMC 5216559. PMID 28057083.
- ↑ Hirst EJ (January 19, 2015), Duchenne muscular dystrophy drug could get OK for U.S. sales in 2016, The Chicago Tribune, archived from the original on May 7, 2017, retrieved February 13, 2017,
has been shown to prolong lives ... a progressive and fatal disease that has no drug treatment available in the US
- ↑ "FDA approves drug to treat Duchenne muscular dystrophy". www.fda.gov. 2017-02-09. Archived from the original on 2017-02-10. Retrieved 2017-02-10.
- ↑ "Marathon Pharmaceuticals to Charge $89,000 for Muscular Dystrophy Drug". www.wsj.com. 2017-02-10. Archived from the original on 2017-02-10. Retrieved 2017-02-10.
- ↑ Walker J, Pulliam S (February 13, 2017), Marathon Pharmaceuticals to Charge $89,000 for Muscular Dystrophy Drug After 70-Fold Increase, The Wall Street Journal, archived from the original on February 10, 2017, retrieved February 13, 2017,
FDA-approved deflazacort treats rare type of disease affecting boys
- ↑ Mukherjee CS (February 10, 2017). "Brainstorm Health Daily". Archived from the original on February 12, 2017. Retrieved February 13, 2017.
- ↑ "PTC Therapeutics Completes Acquisition of Emflaza for the Treatment of Duchenne Muscular Dystrophy in the U.S." PTC Therapeutics, Inc. Archived from the original on 2020-02-16. Retrieved 2021-07-01.
- ↑ "Substâncias: DEFLAZACORT" (in português). Centralx. 2008. Archived from the original on 2018-08-01. Retrieved 2021-07-01. Retrieved on October 28, 2008.
- ↑ "Drugs@FDA: FDA Approved Drug Products". U.S. Food and Drug Administration (FDA). Archived from the original on 2017-06-29. Retrieved 2021-07-01.
- ↑ New Drug Therapy Approvals 2017 (PDF). U.S. Food and Drug Administration (FDA) (Report). January 2018. Archived from the original on 23 October 2020. Retrieved 16 September 2020.
External links
| External sites: |
|
|---|---|
| Identifiers: |