Prednimustine
 | |
| Clinical data | |
|---|---|
| Trade names | Mostarina, Sterecyst |
| Other names | EORTC 1502; Leo 1031; NSC 134087; Prednisolone 21-(4-(4-(bis(2-chloroethyl)-amino)phenyl)butanoate); 11β,17α-Dihydroxy-3,20-dioxopregna-1,4-dien-21-yl 4-{4-[bis(2-chloroethyl)amino]-phenyl}butanoate |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.044.904 |
| Chemical and physical data | |
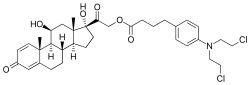
| Formula | C35H45Cl2NO6 |
| Molar mass | 646.65 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Prednimustine, sold under the brand names Mostarina and Sterecyst, is a medication which is used in chemotherapy in the treatment of leukemias and lymphomas.[1][2][3] It is the ester formed from two other drugs, prednisolone and chlorambucil.[1][2][3] Rarely, it has been associated with myoclonus.[4]
See also
References
- 1 2 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 1012–. ISBN 978-1-4757-2085-3.
- 1 2 Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 868–. ISBN 978-3-88763-075-1.
- 1 2 I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 230–. ISBN 978-94-011-4439-1.
- ↑ Monnerat C, Gander M, Leyvraz S (January 1997). "A rare case of prednimustine-induced myoclonus". J. Natl. Cancer Inst. 89 (2): 173–4. doi:10.1093/jnci/89.2.173. PMID 8998190.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.