Floxuridine
 | |
| Names | |
|---|---|
| Other names | 5-fluorodeoxyuridine |
IUPAC name
| |
| Clinical data | |
| Drug class | Antimetabolite[1] |
| Main uses | Gastrointestinal adenocarcinoma, liver cancer, colorectal cancer[1][2] |
| Side effects | Bone marrow suppression, tiredness, headache, dizziness, trouble sleeping, numbness, abdominal pain, constipation, diarrhea, heart burn, nausea, mouth inflammation, rash[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Intra-arterial |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682006 |
| Chemical and physical data | |
| Formula | C9H11FN2O5 |
| Molar mass | 246.194 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 150.5 °C (302.9 °F) |
SMILES
| |
InChI
| |
Floxuridine is a medication used to treat liver cancer and gastrointestinal adenocarcinoma or colorectal cancer that has spread to the liver.[1][2] It is given by gradual injection into an artery supplying the cancer.[1]
Common side effects include bone marrow suppression, tiredness, headache, dizziness, trouble sleeping, numbness, abdominal pain, constipation, diarrhea, heart burn, nausea, mouth inflammation, and rash.[2] Other side effects may include liver problems.[2] Due to the severity of side effects people are generally admitted to hospital during initial treatment.[1] It is an antimetabolite, specifically a pyrimidine analog.[1]
Floxuridine was approved for medical use in the United States in 1970.[1] It is available as a generic medication.[2] In the United States 500 mg did costs about 130 USD; though the only manufacturer stopped making the medication in 2019.[3][4]
Medical uses
The quality of life and survival rates of individuals that receive continuous hepatic artery infusion of floxuridine for colorectal cancer metastases is higher than control groups.[5]
Dosage
It may be given at doses of 0.1 to 0.6 mg/kg/day.[2]
Side effects
Side effects include:[6]
Common
The following occur in more than 30% of people
- Low blood counts. Your white and red blood cells and platelets may temporarily decrease. This can put you at increased risk for infection, anemia and/or bleeding.
- Mouth sores
- Diarrhea (may be severe)
Less common
The following occur in about 10–29% of people
- Poor appetite
- Nausea and vomiting
- Hair loss
- Elevated liver enzymes (temporary increase in alkaline phosphatase, lactate dehydrogenase, transaminase, and bilirubin). This is seen more with the intra-arterial infusion directly into the liver.
- Hand-foot syndrome (Palmar-plantar erythrodysesthesia or PPE) -skin rash, swelling, redness, pain and/or peeling of the skin on the palms of hands and soles of feet
- Stomach ulcers (This is seen more with the intra-arterial infusion).
- Fever of 100.4 °F (38 °C) or higher, chills (possible signs of infection).
- Diarrhea (2 episodes in a 24-hour period)
- Nausea (interferes with ability to eat and unrelieved with prescribed medication)
- Vomiting (vomiting more than 4–5 times in a 24-hour period)
- Mouth sores (painful redness, swelling or ulcers)
- Unusual bleeding or bruising
- Black or tarry stools, or blood in your stools
- Blood in the urine
- Yellowing of the skin or eyes
- Tingling or burning, redness, swelling of the palms of the hands or soles of feet
- Fertility for both men and women may be affected by floxuridine.
Pharmacology
Floxuridine primarily works by stopping the growth of newly born cells. The drug essentially stops DNA from forming in new and rapidly developing cells, which is a sign of a cancerous cell. Therefore, the floxuridine kills the cancerous cells. For colorectal cancer and hepatic metastases, an average adult should be given an Intra-arterial dosage of 0.1–0.6 mg/kg/day as a continuous infusion, continued until intolerable toxicity is reached (white blood cell count <3,500/mm^3 or platelet count <100,000/mm^3).[7] Lethal dosages for other species are below.[8] LD50 is the lethal dose at which half of organisms exposed to the drug die.
| Species | LD50 (mg/kg +/- SE) |
|---|---|
| Mouse | 880 +/- 51 |
| Rat | 670 +/- 73 |
| Rabbit | 94 +/- 19.6 |
| Dog | 157 +/- 46 |
Pharmacodynamics
Floxuridine is a pyrimidine analog that acts as an inhibitor of the S-phase of cell division. This selectively kills rapidly dividing cells. Antimetabolites masquerade as pyrimidine-like molecules which prevents normal pyrimidines from being incorporated into DNA during the S phase of the cell cycle. Fluorouracil (the end-product of catabolism of floxuridine) blocks an enzyme which converts cytosine nucleosides into the deoxy derivative. In addition, DNA synthesis is further inhibited because fluoruracil blocks the incorporation of the thymidine nucleotide into the DNA strand.
Mechanism of action
Floxuridine is rapidly catabolized to 5-fluorouracil, which is the active form of the drug. The primary effect is interference with DNA synthesis and to a lesser extent, inhibition of RNA formation through the drug's incorporation into RNA, thus leading to the production of fraudulent RNA. Fluorouracil also inhibits uracil riboside phosphorylase, which prevents the utilization of preformed uracil in RNA synthesis. As well, the monophosphate of floxuridine, 5-fluoro-2'-deoxyuridine-5'-phosphate (FUDR-MP) inhibits the enzyme thymidylate synthetase. This leads to the inhibition of methylation of deoxyuridylic acid to thymidylic acid, thus interfering with DNA synthesis.
Elimination
The drug is excreted intact and as urea, fluorouracil, α-fluoro-β-ureidopropionic acid, dihydrofluorouracil, α-fluoro-β-guanidopropionic acid and α-fluoro-β-alanine in the urine; it is also expired as respiratory carbon dioxide.
Chemistry
Biosynthesis
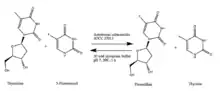
Immobilized Aeromonas salmonicida ATCC 27013, when exposed to thymidine and 5-fluorouracil in phosphate buffer at room temperature for one hour, can synthesize floxuridine and thymine.[9]
History
Floxuridine first gained FDA approval in December 1970 under the brand name FUDR. The drug was initially marketed by Roche, which also did a lot of the initial work on 5-fluorouracil. The National Cancer Institute was an early developer of the drug. Roche sold its FUDR product line in 2001 to F H Faulding, which became Mayne Pharma.
Society and culture
Names
Synonyms for floxuridine include:[10]
|
|
|
Research
Apart from its use in chemotherapy, floxuridine is also used in aging research employing a C. elegans model, namely to stop growth and to prevent reproduction. The latter is brought about by treatment of larvae close to maturity with low doses of floxuridine that, even though allowing normal maturation, causes reproducing individuals to lay eggs that are unable to hatch.[11] This limits the population to a single generation allowing quantification of aging processes and measurement of longevity.[12] It has, however, been indicated that floxuridin exposure by itself increases life expectancy potentially leading to flawed data in respective studies.[13]
In vitro uses of floxuridine include 5-minute treatments of fluorouracil, floxuridine, and mitomycin to increase cell proliferation in Tenon's capsule fibroblasts.[14]
References
- 1 2 3 4 5 6 7 "Floxuridine Monograph for Professionals". Drugs.com. Archived from the original on 16 August 2019. Retrieved 11 December 2021.
- 1 2 3 4 5 6 7 "Floxuridine". LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases. 2012. Archived from the original on 11 December 2021. Retrieved 11 December 2021.
- ↑ "Floxuridine Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 17 April 2021. Retrieved 11 December 2021.
- ↑ "Drug Shortage Detail: Floxuridine Injection". www.ashp.org. Archived from the original on 18 September 2021. Retrieved 11 December 2021.
- ↑ Allen-Mersh TG, Earlam S, Fordy C, Abrams K, Houghton J (November 1994). "Quality of life and survival with continuous hepatic-artery floxuridine infusion for colorectal liver metastases". Lancet. 344 (8932): 1255–60. doi:10.1016/S0140-6736(94)90750-1. PMID 7526096. S2CID 35318063.
- ↑ "Floxuridine". Chemocare. Chemocare.com. Archived from the original on 24 April 2017. Retrieved 17 April 2017.
- ↑ "Floxuridine". Drugs.com. Archived from the original on 2021-01-17. Retrieved 2021-05-07.
- ↑ "Floxuridine". Bedford Laboratories. Archived from the original on 2021-10-31. Retrieved 2021-05-07.
- ↑ Rivero CW, Britos CN, Lozano ME, Sinisterra JV, Trelles JA (June 2012). "Green biosynthesis of floxuridine by immobilized microorganisms". FEMS Microbiology Letters. 331 (1): 31–6. doi:10.1111/j.1574-6968.2012.02547.x. PMID 22428623.
- ↑ Canadian Institutes of Health Research. "Floxuridine". DrugBank. Archived from the original on 11 July 2017. Retrieved 18 April 2017.
- ↑ Hosono, Ryuji (1978-01-01). "Sterilization and growth inhibition of Caenorhabditis elegans by 5-fluorodeoxyuridine". Experimental Gerontology. 13 (5): 369–373. doi:10.1016/0531-5565(78)90047-5. ISSN 0531-5565. PMID 153845. S2CID 46489154. Archived from the original on 2021-10-31. Retrieved 2021-05-07.
- ↑ Gandhi, Schiva; Santelli, John; Mitchell, David H.; Wesley Stiles, J.; Rao Sanadi, D. (February 1980). "A simple method for maintaining large, aging populations of Caenorhabditis elegans". Mechanisms of Ageing and Development. 12 (2): 137–150. doi:10.1016/0047-6374(80)90090-1. PMID 6445025. S2CID 44987472. Archived from the original on 2018-06-16. Retrieved 2021-05-07.
- ↑ Aitlhadj, Layla; Stürzenbaum, Stephen R. (May 2010). "The use of FUdR can cause prolonged longevity in mutant nematodes". Mechanisms of Ageing and Development. 131 (5): 364–365. doi:10.1016/j.mad.2010.03.002. ISSN 1872-6216. PMID 20236608. S2CID 39908205. Archived from the original on 2021-10-31. Retrieved 2021-05-07.
- ↑ Khaw PT, Sherwood MB, MacKay SL, Rossi MJ, Schultz G (August 1992). "Five-minute treatments with fluorouracil, floxuridine, and mitomycin have long-term effects on human Tenon's capsule fibroblasts". Archives of Ophthalmology. 110 (8): 1150–4. doi:10.1001/archopht.1992.01080200130040. PMID 1386726.
External links
| Identifiers: |
|---|