Umbralisib
 | |
| Names | |
|---|---|
| Trade names | Ukoniq |
| Other names | RP5264; TGR-1202 |
IUPAC name
| |
| Clinical data | |
| Main uses | Previously marginal zone lymphoma (MZL) and follicular lymphoma (FL)[1] |
| Side effects | Kidney problems, diarrhea, tiredness, nausea, low neutrophils, muscle pain, low platelets, low red blood cells, upper respiratory tract infection, rash[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Metabolism | CYP2C9, CYP3A4, and CYP1A2[1] |
| Elimination half-life | 91 h[1] |
| Excretion | Feces, urine[1] |
| Chemical and physical data | |
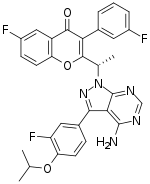
| Formula | C31H24F3N5O3 |
| Molar mass | 571.560 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Umbralisib, sold under the brand name Ukoniq, is a medication which was previously used to treat marginal zone lymphoma (MZL) and follicular lymphoma (FL).[1][3] It was taken by mouth.[1]
Common side effects include kidney problems, diarrhea, tiredness, nausea, low neutrophils, muscle pain, low platelets, low red blood cells, upper respiratory tract infection, and rash.[1] Other side effects may include infections, liver problems, and allergic reactions.[1] Use in pregnancy may harm the baby.[1] It is a kinase inhibitor that blocks PI3K-delta and casein kinase CK1-epsilon.[1]
Umbralisib was approved for medical use in the United States in 2021.[1] However, due to concerns of worsened outcomes it was removed from the market in 2022.[2] Efforts to license in Europe and the United Kingdom have been discontinued.[3]
Medical uses
Umbralisib was indicated for adults with relapsed or refractory marginal zone lymphoma (MZL) who have received at least one prior anti-CD20-based regimen; and adults with relapsed or refractory follicular lymphoma (FL) who have received at least three prior lines of systemic therapy.[1][4][2][5]
On April 15, 2022, TG Therapeutics announced the voluntary withdrawal of Ukoniq (umbralisib) from sale for its approved use in the treatment of marginal zone lymphoma and follicular lymphoma. Furthermore, the company withdrew the pending Biologics License Application (BLA) and supplemental New Drug Application (sNDA) for the treatment of chronic lymphocytic leukemia (CLL) and small lymphocytic leukemia (SLL) which utilized umbralisib in tandem with ublituximab, known as the "U2" regimen. The decision was based on the most recent overall survival (OS) data from the Phase 3 trial, Unity-CLL, that illustrated and increasing imbalance in OS.
Dosage
It was taken at a dose of 800 mg once per day.[1]
Side effects
Side effects include infections, neutropenia, diarrhea and non-infectious colitis, hepatotoxicity, and severe cutaneous reactions.[4]
History
It has undergone clinical studies for chronic lymphocytic leukemia (CLL).[6][7] Three year data (including follicular lymphoma and DLBCL) was announced June 2016.[8] It is in combination trials for various leukemias and lymphomas, such as mantle cell lymphoma (MCL)[9][10] and other lymphomas.[11]
Umbralisib was granted breakthrough therapy designation by the U.S. Food and Drug Administration (FDA) for use in people with marginal zone lymphoma (MZL), a type of cancer with no specifically approved therapies.[12]
FDA approval was based on two single-arm cohorts of an open-label, multi-center, multi-cohort trial, UTX-TGR-205 (NCT02793583), in 69 participants with marginal zone lymphoma (MZL) who received at least one prior therapy, including an anti-CD20 containing regimen, and in 117 participants with follicular lymphoma (FL) after at least two prior systemic therapies.[4] The application for umbralisib was granted priority review for the marginal zone lymphoma (MZL) indication and orphan drug designation for the treatment of MZL and follicular lymphoma (FL).[4][13][14][15][16]
Society and culture
Legal status
In June 2022, due to safety concerns, the U.S. Food and Drug Administration (FDA) withdrew its approval for Ukoniq (umbralisib).[2]
Updated findings from the UNITY-CLL clinical trial show a possible increased risk of death in people receiving Ukoniq.[2] As a result, the FDA determined the risks of treatment with Ukoniq outweigh its benefits.[2] Based upon this determination, the drug's manufacturer, TG Therapeutics, announced it was voluntarily withdrawing Ukoniq from the market for the approved uses in MZL and FL.[2][17]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 "Ukoniq- umbralisib tablet, film coated". DailyMed. Archived from the original on 14 September 2021. Retrieved 13 September 2021.
- 1 2 3 4 5 6 7 "FDA withdrawing cancer drug Ukoniq (umbralisib)". U.S. Food and Drug Administration (FDA). 1 June 2022. Archived from the original on 28 October 2022. Retrieved 1 June 2022.
- 1 2 "Umbralisib". SPS - Specialist Pharmacy Service. 22 June 2020. Archived from the original on 28 November 2021. Retrieved 31 October 2022.
- 1 2 3 4 "FDA grants accelerated approval to umbralisib for marginal zone lymphoma and follicular lymphoma". U.S. Food and Drug Administration (FDA). 5 February 2021. Archived from the original on 5 February 2021. Retrieved 5 February 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "TG Therapeutics Announces Voluntary Withdrawal of the BLA/sNDA for U2 to Treat Patients with CLL and SLL". TG Therapeutics. 15 April 2022.
- ↑ Inman S (19 March 2016). "Novel BTK, PI3K Inhibitors on Horizon for Relapsed CLL". OncLive. Archived from the original on 1 May 2016.
- ↑ "Therapy Focus –- TG Could Benefit From Zydelig Setback". Seeking Alpha. 29 March 2016. Archived from the original on 16 April 2016. Retrieved 19 October 2022.
- ↑ "TG Therapeutics, Inc. Announces First Patient Enrolled in the Registration-Directed UNITY-DLBCL Phase 2b Trial". TG Therapeutics Inc. June 2016. Archived from the original on 2019-04-19. Retrieved 2022-10-19.
- ↑ Clinical trial number NCT02268851 for "A Phase I/Ib Safety and Efficacy Study of the PI3K-delta Inhibitor TGR-1202 and Ibrutinib in Patients With CLL or MCL" at ClinicalTrials.gov
- ↑ "Follow-Up Data for Combination of TGR-1202 (umbralisib) plus Ibrutinib in Patients with Relapsed or Refractory CLL and MCL" (Press release). TG Therapeutics. 14 June 2017. Archived from the original on 20 October 2021. Retrieved 19 October 2022 – via Globenewswire.
- ↑ Clinical trial number NCT02793583 for "Study to Assess the Efficacy and Safety of Ublituximab + TGR-1202 With or Without Bendamustine and TGR-1202 Alone in Patients With Previously Treated Non-Hodgkin's Lymphoma (UNITY-NHL)" at ClinicalTrials.gov
- ↑ Columbus G (22 January 2019). "FDA Grants Umbralisib Breakthrough Designation for Marginal Zone Lymphoma". OncLive. Archived from the original on 23 January 2019.
- ↑ "Orphan Treatment of extranodal marginal zone lymphoma". U.S. Food and Drug Administration (FDA). 11 April 2019. Archived from the original on 28 October 2022. Retrieved 5 February 2021.
- ↑ "Orphan Treatment of splenic marginal zone lymphoma". U.S. Food and Drug Administration (FDA). 11 April 2019. Archived from the original on 25 February 2021. Retrieved 5 February 2021.
- ↑ "Orphan Treatment of Follicular Lymphoma". U.S. Food and Drug Administration (FDA). 11 April 2019. Archived from the original on 14 February 2021. Retrieved 5 February 2021.
- ↑ "Orphan Treatment of nodal marginal zone lymphoma". U.S. Food and Drug Administration (FDA). 11 April 2019. Archived from the original on 25 February 2021. Retrieved 5 February 2021.
- ↑ "TG Therapeutics Announces Voluntary Withdrawal of the BLA/sNDA for U2 to Treat Patients with CLL and SLL" (Press release). TG Therapeutics. 15 April 2022. Archived from the original on 1 June 2022. Retrieved 1 June 2022.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- "Umbralisib". NCI Drug Dictionary. National Cancer Institute. Archived from the original on 2022-05-16. Retrieved 2022-10-19.