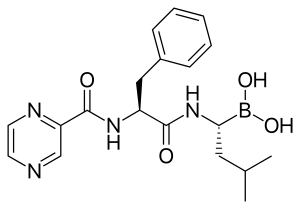
Bortezomib
 | |
 | |
| Names | |
|---|---|
| Trade names | Velcade, Chemobort, Bortecad, others |
| Other names | PS-341 |
IUPAC name
| |
| Clinical data | |
| Drug class | Anti-cancer medication[1] |
| Main uses | Multiple myeloma, mantle cell lymphoma[1] |
| Side effects | Nausea, diarrhea, tiredness, low platelets, fever, numbness, low white blood cells, shortness of breath, rash, abdominal pain[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Subcutaneous, IV |
| Defined daily dose | Not established[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607007 |
| Legal | |
| License data |
|
| Legal status |
|
| Pharmacokinetics | |
| Protein binding | 83% |
| Metabolism | Liver, CYP extensively involved |
| Elimination half-life | 9 to 15 hours |
| Chemical and physical data | |
| Formula | C19H25BN4O4 |
| Molar mass | 384.24 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Bortezomib, sold under the brand name Velcade among others, is an anti-cancer medication used to treat multiple myeloma and mantle cell lymphoma.[1] This includes multiple myeloma in those who have and have not previously received treatment.[3] It is generally used together with other medications.[3] It is given by injection.[1]
Common side effects include nausea, diarrhea, tiredness, low platelets, fever, numbness, low white blood cells, shortness of breath, rash, and abdominal pain.[1] Other severe side effects include low blood pressure, tumour lysis syndrome, heart failure, and reversible posterior leukoencephalopathy syndrome.[1][3] It is in the class of medications known as proteasome inhibitor.[1] It works by inhibiting proteasomes, cellular complexes that break down proteins.[3]
Bortezomib was approved for medical use in the United States in 2003 and in Europe in 2004.[1][3] It is on the World Health Organization's List of Essential Medicines.[4] In the United States it costs US$1,360 per 3.5 mg vial.[5] In the United Kingdom this amount costs the NHS £762 such that a course of treatment is about £12,261 as of 2014.[6]
Medical use
Two open-label trials established the efficacy of bortezomib (with or without dexamethasone) on days 1,4,8, and 11 of a 21-day cycle for a maximum of eight cycles in heavily pretreated people with relapsed/refractory multiple myeloma.[7] The phase III demonstrated the superiority of bortezomib over a high-dose dexamethasone regimen (e.g. median TTP 6.2 vs 3.5 months, and 1-year survival 80% vs 66%).[7] New studies show that bortezomib may potentially help recover from vincristine treatment in treating acute lymphoblastic leukemia, when replacing vincristine in the process.[8]
As of 2019 continuous bortezomib, lenalidomide, and dexamethasone (VRDc) works well for people with multiple myeloma who are not able to receive a transplant.[9]
Dosage
The defined daily dose is not established.[2]
Side effects
Gastro-intestinal (GI) effects and asthenia are the most common side effects.[11] Bortezomib is associated with peripheral neuropathy in 30% of people resulting in pain. This can be worse in patients with pre-existing neuropathy. In addition, myelosuppression causing neutropenia and thrombocytopenia can also occur and be dose-limiting. However, these side effects are usually mild relative to bone marrow transplantation and other treatment options for patients with advanced disease. Bortezomib is associated with a high rate of shingles,[12] although prophylactic acyclovir can reduce the risk of this.[13]
Ocular side effects such as chalazion or hordeolum (stye) may be more common in women and have led to discontinuation of treatment.[14] Acute interstitial nephritis has also been reported.[15]
Interactions
Polyphenols derived from green tea extract including epigallocatechin gallate (EGCG), which were expected to have a synergistic effect, instead were found to reduce the effectiveness of bortezomib in cell culture experiments.[16]
Pharmacology
Structure
The drug is an N-protected dipeptide and can be written as Pyz-Phe-boroLeu, which stands for pyrazinoic acid, phenylalanine and Leucine with a boronic acid instead of a carboxylic acid.
Mechanism
The boron atom in bortezomib binds the catalytic site of the 26S proteasome[17] with high affinity and specificity. In normal cells, the proteasome regulates protein expression and function by degradation of ubiquitylated proteins, and also rids the cell of abnormal or misfolded proteins. Clinical and preclinical data support a role for the proteasome in maintaining the immortal phenotype of myeloma cells, and cell-culture and xenograft data support a similar function in solid tumor cancers. While multiple mechanisms are likely to be involved, proteasome inhibition may prevent degradation of pro-apoptotic factors, thereby triggering programmed cell death in neoplastic cells. Recently, it was found that bortezomib caused a rapid and dramatic change in the levels of intracellular peptides that are produced by the proteasome.[18] Some intracellular peptides have been shown to be biologically active, and so the effect of bortezomib on the levels of intracellular peptides may contribute to the biological and/or side effects of the drug.
Pharmacokinetics
After subcutaneous administration, peak plasma levels are ~25-50 nM and this peak is sustained for 1-2 hrs. After intravenous injection, peak plasma levels are ~500 nM but only for ~5 minutes, after which the levels rapidly drop as the drug distributes to tissues (volume of distribution is ~500 L).[19][20] Both routes provide equal drug exposures and generally comparable therapeutic efficacy. Elimination half life is 9–15 hours and the drug is primarily cleared by hepatic metabolism.[21]
The pharmacodynamics of bortezomib are determined by quantifying proteasome inhibition in peripheral blood mononuclear cells taken from patients receiving the drug.
History
Bortezomib was originally made in 1995 at Myogenics. The drug (PS-341) was tested in a small Phase I clinical trial on patients with multiple myeloma. It was brought to further clinical trials by Millennium Pharmaceuticals in October 1999.[22]
In May 2003, seven years after the initial synthesis, bortezomib (marketed as Velcade by Millennium Pharmaceuticals Inc.) was approved in the United States by the U.S. Food and Drug Administration (FDA) for use in multiple myeloma, based on the results from the SUMMIT Phase II trial.[23][24] In 2008, bortezomib was approved in the United States for initial treatment of patients with multiple myeloma.[25] Bortezomib was previously approved in 2005, for the treatment of patients with multiple myeloma who had received at least one prior therapy and in 2003, for the treatment of more refractory multiple myeloma.[25]
The 2008 approval was based on an international, multicenter, open label, active-control trial in previously untreated patients with symptomatic multiple myeloma.[25] Patients were randomized to receive either nine cycles of oral melphalan (M) plus prednisone (P) or MP plus bortezomib.[25] Patients received M (9 mg/m2 ) plus prednisone (60 mg/m2 ) daily for four days every 6 weeks or the same MP schedule with bortezomib, 1.3 mg/m2 iv on days 1, 8, 11, 22, 25, 29, and 32 of every 6 week cycle for 4 cycles then once weekly for 4 weeks for 5 cycles.[25] Time- to- progression (TTP) was the primary efficacy endpoint.[25] Overall survival (OS), progression-free survival (PFS), and response rate (RR) were secondary endpoints.[25] Eligible patients were age > 65 years.[25] A total of 682 patients were randomized: 338 to receive MP and 344 to the combination of bortezomib plus MP.[25] Demographics and baseline disease characteristics were similar between the two groups.[25]
The trial was stopped following a pre-specified interim analysis showing a statistically significant improvement in TTP with the addition of bortezomib to MP (median 20.7 months) compared with MP (median 15 months) [HR: 0.54 (95% CI: 0.42, 0.70), p= 0.000002].[25] OS, PFS, and RR also were significantly superior for the bortezomib-MP combination.[25]
In August 2014, bortezomib was approved in the United States for the retreatment of adult patients with multiple myeloma[26][27] who had previously responded to Velcade therapy and relapsed at least six months following completion of prior treatment.[27]
In October 2014, bortezomib was approved in the United States for the treatment of treatment-naïve patients with mantle cell lymphoma (MCL).[27]
Costs
In the UK, NICE initially recommended against Velcade in October 2006 due to its cost of about £18,000 per person, and because studies reviewed by NICE reported that it could only extend the life expectancy by an average of six months over standard treatment.[28] However, the company later proposed a performance-linked cost reduction for multiple myeloma,[29] and this was accepted.[30]
See also
- Ixazomib, a proteasome inhibitor that is given by mouth
References
- 1 2 3 4 5 6 7 8 9 "Bortezomib Monograph for Professionals". Drugs.com. Archived from the original on 13 October 2019. Retrieved 13 October 2019.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 20 September 2020. Retrieved 11 September 2020.
- 1 2 3 4 5 "Velcade". European Medicines Agency. 17 September 2018. Archived from the original on 13 October 2019. Retrieved 13 October 2019.
- ↑ World Health Organization (2019). "World Health Organization model list of essential medicines: 21st list 2019". World Health Organization (WHO). hdl:10665/325771.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ "Bortezomib Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 13 October 2019. Retrieved 13 October 2019.
- ↑ "Bortezomib for induction therapy in multiple myeloma before high-dose chemotherapy and autologous stem cell transplantation". NICE. February 2014. p. 2. Archived from the original on 13 October 2019. Retrieved 13 October 2019.
- 1 2 Curran M, McKeage K (2009). "Bortezomib: A Review of its Use in Patients with Multiple Myeloma". Drugs. 69 (7): 859–888. doi:10.2165/00003495-200969070-00006. PMID 19441872. Archived from the original on 2011-10-08. Retrieved 2010-03-26.
- ↑ Joshi, Jaitri, et al. “Switching to Bortezomib May Improve Recovery From Severe Vincristine Neuropathy in Pediatric Acute Lymphoblastic Leukemia.” Journal of Pediatric Hematology/Oncology, vol. 41, no. 6, Aug. 2019, pp. 457–462., doi:10.1097/mph.0000000000001529.
- ↑ Piechotta, Vanessa; Jakob, Tina; Langer, Peter; Monsef, Ina; Scheid, Christof; Estcourt, Lise J; Ocheni, Sunday; Theurich, Sebastian; Kuhr, Kathrin; Scheckel, Benjamin; Adams, Anne (2019-11-25). Cochrane Haematology Group (ed.). "Multiple drug combinations of bortezomib, lenalidomide, and thalidomide for first-line treatment in adults with transplant-ineligible multiple myeloma: a network meta-analysis". Cochrane Database of Systematic Reviews. doi:10.1002/14651858.CD013487. Archived from the original on 2021-08-28. Retrieved 2021-02-18.
- ↑ Herrmann, Joerg (11 January 2022). Cardio-Oncology Practice Manual: A Companion to Braunwald’s Heart Disease E-Book. Elsevier Health Sciences. p. 488. ISBN 978-0-323-68136-0. Archived from the original on 12 February 2022. Retrieved 12 February 2022.
- ↑ Highlights Of Prescribing Information Archived February 19, 2009, at the Wayback Machine
- ↑ Oakervee HE, Popat R, Curry N, et al. (2005). "PAD combination therapy (PS-341/bortezomib, doxorubicin and dexamethasone) for previously untreated patients with multiple myeloma". Br J Haematol. 129 (6): 755–62. doi:10.1111/j.1365-2141.2005.05519.x. PMID 15953001.
- ↑ Pour L.; Adam Z.; Buresova L.; et al. (2009). "Varicella-zoster virus prophylaxis with low-dose acyclovir in patients with multiple myeloma treated with bortezomib". Clinical Lymphoma & Myeloma. 9 (2): 151–3. doi:10.3816/CLM.2009.n.036. PMID 19406726.
- ↑ Dennis, Michael; Maoz, Asaf; Hughes, David; Sanchorawala, Vaishali; Sloan, J. Mark; Sarosiek, Shayna (2019). "Bortezomib ocular toxicities: Outcomes with ketotifen". American Journal of Hematology. 0 (3): E80–E82. doi:10.1002/ajh.25382. ISSN 1096-8652. PMID 30575098.
- ↑ Cheungpasitporn, Wisit; Leung, Nelson; Rajkumar, S. Vincent; Cornell, Lynn D.; Sethi, Sanjeev; Angioi, Andrea; Fervenza, Fernando C. (2015). "Bortezomib-induced acute interstitial nephritis". Nephrol. Dial. Transplant. 30 (7): 1225–1229. doi:10.1093/ndt/gfv222. PMID 26109684.
- ↑ Golden, EB; Lam, PY; Kardosh, A; Gaffney, KJ; Cadenas, E; Louie, SG; Petasis, NA; Chen, TC; Schönthal, AH (4 June 2009). "Green tea polyphenols block the anticancer effects of bortezomib and other boronic acid-based proteasome inhibitors". Blood. 113 (23): 5927–37. doi:10.1182/blood-2008-07-171389. PMID 19190249.
- ↑ Bonvini P, Zorzi E, Basso G, Rosolen A (2007). "Bortezomib-mediated 26S proteasome inhibition causes cell-cycle arrest and induces apoptosis in CD-30+ anaplastic large cell lymphoma". Leukemia. 21 (4): 838–42. doi:10.1038/sj.leu.2404528. PMID 17268529.
- ↑ Gelman JS, Sironi J, Berezniuk I, Dasgupta S, Castro LM, Gozzo FC, Ferro ES, Fricker LD (2013). "Alterations of the intracellular peptidome in response to the proteasome inhibitor bortezomib". PLOS ONE. 8 (1): e53263. Bibcode:2013PLoSO...853263G. doi:10.1371/journal.pone.0053263. PMC 3538785. PMID 23308178.
- ↑ Reece DE, Sullivan D, Lonial S, Mohrbacher AF, Chatta G, Shustik C, Burris H 3rd, Venkatakrishnan K, Neuwirth R, Riordan WJ, Karol M, von Moltke LL, Acharya M, Zannikos P, Keith Stewart A (2011). "Pharmacokinetic and pharmacodynamic study of two doses of bortezomib in patients with relapsed multiple myeloma". Cancer Chemother. Pharmacol. 67 (1): 57–67. doi:10.1007/s00280-010-1283-3. PMC 3951913. PMID 20306195.
- ↑ Voorhees PM, Dees EC, O'Neil B, Orlowski RZ (2003). "The proteasome as a target for cancer therapy". Clin Cancer Res. 9 (17): 6316–25. PMID 14695130.
- ↑ Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M, Rekhtman G, Masliak Z, Robak T, Shubina A, Arnulf B, Kropff M, Cavet J, Esseltine DL, Feng H, Girgis S, van de Velde H, Deraedt W, Harousseau JL (2011). "Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study". The Lancet Oncology. 12 (5): 431–40. doi:10.1016/s1470-2045(11)70081-x. PMID 21507715.
- ↑ Larkin, Marilynn (November 1999). "(In)famous trials brought to life". The Lancet. 354 (9193): 1915. doi:10.1016/s0140-6736(05)76886-0. ISSN 0140-6736.
- ↑ Adams J, Kauffman M (2004). "Development of the Proteasome Inhibitor Velcade (Bortezomib)". Cancer Invest. 22 (2): 304–11. doi:10.1081/CNV-120030218. PMID 15199612.
- ↑ "Drug Approval Package: Velcade (Bortezomib) NDA #021602". U.S. Food and Drug Administration (FDA). 13 May 2003. Archived from the original on 5 December 2019. Retrieved 5 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - 1 2 3 4 5 6 7 8 9 10 11 12 "Velcade (bortezomib) is Approved for Initial Treatment of Patients with Multiple Myeloma" (Press release). U.S. Food and Drug Administration (FDA). June 23, 2008. Archived from the original on 1 December 2011. Retrieved 5 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Millennium: The Takeda Oncology Company". .millennium.com. 2014-08-08. Archived from the original on 2018-11-01.
- 1 2 3 Raedler L (March 2015). "Velcade (Bortezomib) Receives 2 New FDA Indications: For Retreatment of Patients with Multiple Myeloma and for First-Line Treatment of Patients with Mantle-Cell Lymphoma". Am Health Drug Benefits. 8 (Spec Feature): 135–40. PMC 4665054. PMID 26629279.
- ↑ "NHS watchdog rejects cancer drug". BBC News UK. 2006-10-20. Archived from the original on 2006-11-07. Retrieved 2009-08-14.
- ↑ "Summary of VELCADE Response Scheme" (PDF). Archived from the original (PDF) on 2009-04-19. Retrieved 2009-08-14.
- ↑ "More Velcade-Style Risk-Sharing In The UK?". Euro Pharma Today. 2009-01-21. Archived from the original on 2011-07-10. Retrieved 2009-08-14.
External links
| External sites: |
|
|---|---|
| Identifiers: |