Tipifarnib
 | |
| Clinical data | |
|---|---|
| Other names | R115777 |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
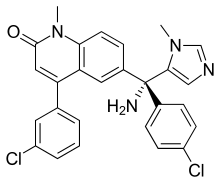
| Formula | C27H22Cl2N4O |
| Molar mass | 489.40 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Tipifarnib (INN,[1]: 213 proposed trade name Zarnestra) is a farnesyltransferase inhibitor. Farnesyltransferase inhibitors block the activity of the farnesyltransferase enzyme by inhibiting prenylation of the CAAX tail motif, which ultimately prevents Ras from binding to the membrane, rendering it inactive.[2]
History
The compound was discovered by Johnson & Johnson Pharmaceutical Research & Development, L.L.C, with registration number R115777.
For treatment of progressive plexiform neurofibromas associated with neurofibromatosis type I, it passed phase I clinical trials but was suspended (NCT00029354) in phase II.[3][4]
Tipifarnib was submitted to the FDA by Johnson & Johnson for the treatment of AML in patients aged 65 and over with a new drug application (NDA) to the FDA on January 24, 2005. In June 2005, the FDA issued a "not approvable" letter for tipifarnib.[5]
Kura Oncology in-licensed tipifarnib from Janssen in 2014.[6]
Investigations
Cancer
The inhibitor is being investigated in patients with HRAS mutant head and neck cancer, peripheral T-cell lymphoma (PTCL), myelodysplastic syndromes (MDS), and chronic myelomonocytic leukemia (CMML).[7][8][9][10][11] It was previously tested in clinical trials in patients in certain stages of breast cancer.[12] It was also investigated as a treatment for multiple myeloma.[13]
Progeria

It was shown on a mouse model of Hutchinson–Gilford progeria syndrome that dose-dependent administration of tipifarnib can significantly prevent both the onset of the cardiovascular phenotype as well as the late progression of existing cardiovascular disease.[14]
References
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names (Rec. INN): List 46" (PDF). World Health Organization. Retrieved 16 November 2016.
- ↑ Cox AD, Der CJ, Philips MR (April 2015). "Targeting RAS Membrane Association: Back to the Future for Anti-RAS Drug Discovery?". Clinical Cancer Research. 21 (8): 1819–27. doi:10.1158/1078-0432.CCR-14-3214. PMC 4400837. PMID 25878363.
- ↑ Clinical trial number NCT00025454 for "R115777 in Treating Patients With Advanced Solid Tumors" at ClinicalTrials.gov
- ↑ Clinical trial number NCT00029354 for "R115777 to Treat Children With Neurofibromatosis Type 1 and Progressive Plexiform Neurofibromas" at ClinicalTrials.gov
- ↑ "Johnson & Johnson Pharmaceutical Research & Development, L.L.C. Receives Not Approvable Letter From FDA for Tipifarnib Based on Phase II Data". PR Newswire. Jun 30, 2005. Retrieved 16 November 2016.
- ↑ Carroll J (12 March 2015). "Kura sheds stealth mode with $60M for PhII cancer drug licensed from J&J | FierceBiotech". www.fiercebiotech.com.
- ↑ Witzig TE, Tang H, Micallef IN, Ansell SM, Link BK, Inwards DJ, et al. (November 2011). "Multi-institutional phase 2 study of the farnesyltransferase inhibitor tipifarnib (R115777) in patients with relapsed and refractory lymphomas". Blood. 118 (18): 4882–9. doi:10.1182/blood-2011-02-334904. PMC 3208296. PMID 21725056.
- ↑ Clinical trial number NCT02383927 for "Phase II Study of Tipifarnib in Squamous Head and Neck Cancer With HRAS Mutations" at ClinicalTrials.gov
- ↑ Clinical trial number NCT02464228 for "Study of Tipifarnib in Subjects With Relapsed or Refractory Peripheral T-Cell Lymphoma (PTCL)" at ClinicalTrials.gov
- ↑ Clinical trial number NCT02779777 for "Tipifarnib in Subjects With Myelodysplastic Syndromes" at ClinicalTrials.gov
- ↑ Clinical trial number NCT02807272 for "Tipifarnib in Subjects With Chronic Myelomonocytic Leukemia (CMML)" at ClinicalTrials.gov
- ↑ Sparano JA, Moulder S, Kazi A, Coppola D, Negassa A, Vahdat L, et al. (April 2009). "Phase II trial of tipifarnib plus neoadjuvant doxorubicin-cyclophosphamide in patients with clinical stage IIB-IIIC breast cancer". Clinical Cancer Research. 15 (8): 2942–8. doi:10.1158/1078-0432.CCR-08-2658. PMC 2785076. PMID 19351752.
- ↑ Alsina M, Fonseca R, Wilson EF, Belle AN, Gerbino E, Price-Troska T, et al. (May 2004). "Farnesyltransferase inhibitor tipifarnib is well tolerated, induces stabilization of disease, and inhibits farnesylation and oncogenic/tumor survival pathways in patients with advanced multiple myeloma". Blood. 103 (9): 3271–7. doi:10.1182/blood-2003-08-2764. PMID 14726402.
- ↑ Capell BC, Olive M, Erdos MR, Cao K, Faddah DA, Tavarez UL, et al. (October 2008). "A farnesyltransferase inhibitor prevents both the onset and late progression of cardiovascular disease in a progeria mouse model" (PDF). Proceedings of the National Academy of Sciences of the United States of America. 105 (41): 15902–7. Bibcode:2008PNAS..10515902C. doi:10.1073/pnas.0807840105. PMC 2562418. PMID 18838683.