Streptomyces
| Streptomyces | |
|---|---|
 | |
| Slide culture of a Streptomyces species | |
| Scientific classification | |
| Kingdom: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | Streptomyces Waksman and Henrici 1943 (Approved Lists 1980) |
| Diversity | |
| About 550 species | |
| Synonyms[1] | |
| |

Streptomyces is the largest genus of Actinobacteria and the type genus of the family Streptomycetaceae.[3] Over 500 species of Streptomyces bacteria have been described.[4] As with the other Actinobacteria, streptomycetes are gram-positive, and have genomes with high GC content.[5] Found predominantly in soil and decaying vegetation, most streptomycetes produce spores, and are noted for their distinct "earthy" odor that results from production of a volatile metabolite, geosmin.
Streptomycetes are characterised by a complex secondary metabolism.[5] They produce over two-thirds of the clinically useful antibiotics of natural origin (e.g., neomycin, cypemycin, grisemycin, bottromycins and chloramphenicol).[6][7] The antibiotic streptomycin takes its name directly from Streptomyces. Streptomycetes are infrequent pathogens, though infections in humans, such as mycetoma, can be caused by S. somaliensis and S. sudanensis, and in plants can be caused by S. caviscabies, S. acidiscabies, S. turgidiscabies and S. scabies.
Taxonomy
Streptomyces is the type genus of the family Streptomycetaceae[8] and currently covers close to 576 species with the number increasing every year.[9] Acidophilic and acid-tolerant strains that were initially classified under this genus have later been moved to Kitasatospora (1997) [10] and Streptacidiphilus (2003).[11] Species nomenclature are usually based on their color of hyphae and spores.
Saccharopolyspora erythraea was formerly placed in this genus (as Streptomyces erythraeus).
Morphology
The genus Streptomyces includes aerobic, Gram-positive, filamentous bacteria that produce well-developed vegetative hyphae (between 0.5-2.0 µm in diameter) with branches. They form a complex substrate mycelium that aids in scavenging organic compounds from their substrates.[12] Although the mycelia and the aerial hyphae that arise from them are amotile, mobility is achieved by dispersion of spores.[12] Spore surfaces may be hairy, rugose, smooth, spiny or warty.[13] In some species, aerial hyphae consist of long, straight filaments, which bear 50 or more spores at more or less regular intervals, arranged in whorls (verticils). Each branch of a verticil produces, at its apex, an umbel, which carries from two to several chains of spherical to ellipsoidal, smooth or rugose spores.[12] Some strains form short chains of spores on substrate hyphae. Sclerotia-, pycnidia-, sporangia-, and synnemata-like structures are produced by some strains.
Genomics
The complete genome of "S. coelicolor strain A3(2)" was published in 2002.[14] At the time, the "S. coelicolor" genome was thought to contain the largest number of genes of any bacterium.[14] The chromosome is 8,667,507 bp long with a GC-content of 72.1%, and is predicted to contain 7,825 protein-encoding genes.[14] In terms of taxonomy, "S. coelicolor A3(2)" belongs to the species S. violaceoruber, and is not a validly described separate species; "S. coelicolor A3(2)" is not to be mistaken for the actual S. coelicolor (Müller), although it is often referred to as S. coelicolor for convenience.[15] The transcriptome and translatome analyses of the strain A3(2) were published in 2016.[16]
The first complete genome sequence of S. avermitilis was completed in 2003.[17] Each of these genomes forms a chromosome with a linear structure, unlike most bacterial genomes, which exist in the form of circular chromosomes.[18] The genome sequence of S. scabies, a member of the genus with the ability to cause potato scab disease, has been determined at the Wellcome Trust Sanger Institute. At 10.1 Mbp long and encoding 9,107 provisional genes, it is the largest known Streptomyces genome sequenced, probably due to the large pathogenicity island.[18][19]
Biotechnology
In recent years, biotechnology researchers have begun using Streptomyces species for heterologous expression of proteins. Traditionally, Escherichia coli was the species of choice to express eukaryotic genes, since it was well understood and easy to work with.[20][21] Expression of eukaryotic proteins in E. coli may be problematic. Sometimes, proteins do not fold properly, which may lead to insolubility, deposition in inclusion bodies, and loss of bioactivity of the product.[22] Though E. coli strains have secretion mechanisms, these are of low efficiency and result in secretion into the periplasmic space, whereas secretion by a Gram-positive bacterium such as a Streptomyces species results in secretion directly into the extracellular medium. In addition, Streptomyces species have more efficient secretion mechanisms than E.coli. The properties of the secretion system is an advantage for industrial production of heterologously expressed protein because it simplifies subsequent purification steps and may increase yield. These properties among others make Streptomyces spp. an attractive alternative to other bacteria such as E. coli and Bacillus subtilis.[22]
Plant pathogenic bacteria
So far, ten species belonging to this genus have been found to be pathogenic to plants:[9]
- S. scabiei
- S. acidiscabies
- S. europaeiscabiei
- S. luridiscabiei
- S. niveiscabiei
- S. puniciscabiei
- S. reticuliscabiei
- S. stelliscabiei
- S. turgidiscabies (scab disease in potatoes)
- S. ipomoeae (soft rot disease in sweet potatoes)
Medicine
Streptomyces is the largest antibiotic-producing genus, producing antibacterial, antifungal, and antiparasitic drugs, and also a wide range of other bioactive compounds, such as immunosuppressants.[23] Almost all of the bioactive compounds produced by Streptomyces are initiated during the time coinciding with the aerial hyphal formation from the substrate mycelium.[12]
Antifungals
Streptomycetes produce numerous antifungal compounds of medicinal importance, including nystatin (from S. noursei), amphotericin B (from S. nodosus),[24] and natamycin (from S. natalensis).
Antibacterials
Members of the genus Streptomyces are the source for numerous antibacterial pharmaceutical agents; among the most important of these are:
- Chloramphenicol (from S. venezuelae)[25]
- Daptomycin (from S. roseosporus)[26]
- Fosfomycin (from S. fradiae)[27]
- Lincomycin (from S. lincolnensis)[28]
- Neomycin (from S. fradiae)[29]
- Nourseothricin
- Puromycin (from S. alboniger)[30]
- Streptomycin (from S. griseus)[31]
- Tetracycline (from S. rimosus and S. aureofaciens)[32]
- Oleandomycin (from S. antibioticus)[33][34][35]
- Tunicamycin (from S. torulosus)[36]
- Mycangimycin (from Streptomyces sp. SPB74 and S. antibioticus)[37]
- Boromycin (from S. antibioticus)[38]
- Bambermycin (from S. bambergiensis and S. ghanaensis, the active compound being moenomycins A and C)[39]
Clavulanic acid (from S. clavuligerus) is a drug used in combination with some antibiotics (like amoxicillin) to block and/or weaken some bacterial-resistance mechanisms by irreversible beta-lactamase inhibition. Novel antiinfectives currently being developed include Guadinomine (from Streptomyces sp. K01-0509),[40] a compound that blocks the Type III secretion system of Gram-negative bacteria.
Antiparasitic drugs
S. avermitilis is responsible for the production of one of the most widely employed drugs against nematode and arthropod infestations, ivermectin.
Other
Less commonly, streptomycetes produce compounds used in other medical treatments: migrastatin (from S. platensis) and bleomycin (from S. verticillus) are antineoplastic (anticancer) drugs; boromycin (from S. antibioticus) exhibits antiviral activity against the HIV-1 strain of HIV, as well as antibacterial activity. Staurosporine (from S. staurosporeus) also has a range of activities from antifungal to antineoplastic (via the inhibition of protein kinases).
S. hygroscopicus and S. viridochromogenes produce the natural herbicide bialaphos.
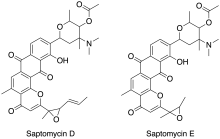
Saptomycins are chemical compounds isolated from Streptomyces.[41]
See also
- Antimycin A – Chemical compound produced by this bacterium used in piscicides
- Geosmin – Chemical compound responsible for the characteristic odour of earth
- Streptomyces isolates
References
- ↑ Euzéby JP, Parte AC. "Streptomyces". List of Prokaryotic names with Standing in Nomenclature (LPSN). Retrieved June 9, 2021.
- ↑ Van der Meij, A., Willemse, J., Schneijderberg, M.A., Geurts, R., Raaijmakers, J.M. and van Wezel, G.P. (2018) "Inter-and intracellular colonization of Arabidopsis roots by endophytic actinobacteria and the impact of plant hormones on their antimicrobial activity". Antonie van Leeuwenhoek, 111(5): 679–690. doi:10.1007/s10482-018-1014-z
- ↑ Kämpfer P (2006). "The Family Streptomycetaceae, Part I: Taxonomy". In Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E (eds.). The Prokaryotes. pp. 538–604. doi:10.1007/0-387-30743-5_22. ISBN 978-0-387-25493-7.
- ↑ Euzéby JP (2008). "Genus Streptomyces". List of Prokaryotic names with Standing in Nomenclature. Retrieved 2008-09-28.
- 1 2 Madigan M, Martinko J, eds. (2005). Brock Biology of Microorganisms (11th ed.). Prentice Hall. ISBN 978-0-13-144329-7.
- ↑ Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA (2000). Practical Streptomyces Genetics (2nd ed.). Norwich, England: John Innes Foundation. ISBN 978-0-7084-0623-6.
- ↑ Bibb MJ (December 2013). "Understanding and manipulating antibiotic production in actinomycetes". Biochemical Society Transactions. 41 (6): 1355–64. doi:10.1042/BST20130214. PMID 24256223.
- ↑ Anderson AS, Wellington EM (May 2001). "The taxonomy of Streptomyces and related genera". International Journal of Systematic and Evolutionary Microbiology. 51 (Pt 3): 797–814. doi:10.1099/00207713-51-3-797. PMID 11411701.
- 1 2 Labeda DP (October 2011). "Multilocus sequence analysis of phytopathogenic species of the genus Streptomyces". International Journal of Systematic and Evolutionary Microbiology. 61 (Pt 10): 2525–2531. doi:10.1099/ijs.0.028514-0. PMID 21112986.
- ↑ Zhang Z, Wang Y, Ruan J (October 1997). "A proposal to revive the genus Kitasatospora (Omura, Takahashi, Iwai, and Tanaka 1982)". International Journal of Systematic Bacteriology. 47 (4): 1048–54. doi:10.1099/00207713-47-4-1048. PMID 9336904.
- ↑ Kim SB, Lonsdale J, Seong CN, Goodfellow M (2003). "Streptacidiphilus gen. nov., acidophilic actinomycetes with wall chemotype I and emendation of the family Streptomycetaceae (Waksman and Henrici (1943)AL) emend. Rainey et al. 1997". Antonie van Leeuwenhoek. 83 (2): 107–16. doi:10.1023/A:1023397724023. PMID 12785304. S2CID 12901116.
- 1 2 3 4 Chater K, Losick R (1984). "Morphological and physiological differentiation in Streptomyces". Microbial development. Vol. 16. pp. 89–115. doi:10.1101/087969172.16.89 (inactive 31 October 2021). ISBN 978-0-87969-172-1. Retrieved 2012-01-19.
{{cite book}}: CS1 maint: DOI inactive as of October 2021 (link) - ↑ Dietz A, Mathews J (March 1971). "Classification of Streptomyces spore surfaces into five groups". Applied Microbiology. 21 (3): 527–33. doi:10.1128/AEM.21.3.527-533.1971. PMC 377216. PMID 4928607.
- 1 2 3 Bentley SD, Chater KF, Cerdeño-Tárraga AM, Challis GL, Thomson NR, James KD, et al. (May 2002). "Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2)". Nature. 417 (6885): 141–7. Bibcode:2002Natur.417..141B. doi:10.1038/417141a. PMID 12000953. S2CID 4430218.
- ↑ Chater KF, Biró S, Lee KJ, Palmer T, Schrempf H (March 2010). "The complex extracellular biology of Streptomyces". FEMS Microbiology Reviews. 34 (2): 171–98. doi:10.1111/j.1574-6976.2009.00206.x. PMID 20088961.
- ↑ Jeong Y, Kim JN, Kim MW, Bucca G, Cho S, Yoon YJ, et al. (June 2016). "The dynamic transcriptional and translational landscape of the model antibiotic producer Streptomyces coelicolor A3(2)". Nature Communications. 7 (1): 11605. Bibcode:2016NatCo...711605J. doi:10.1038/ncomms11605. PMC 4895711. PMID 27251447.
- ↑ Ikeda H, Ishikawa J, Hanamoto A, Shinose M, Kikuchi H, Shiba T, et al. (May 2003). "Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis". Nature Biotechnology. 21 (5): 526–31. doi:10.1038/nbt820. PMID 12692562.
- 1 2 Dyson P (1 January 2011). Streptomyces: Molecular Biology and Biotechnology. Horizon Scientific Press. p. 5. ISBN 978-1-904455-77-6. Retrieved 16 January 2012.
- ↑ "Streptomyces scabies". Sanger Institute. Retrieved 2001-02-26.
- ↑ Brawner M, Poste G, Rosenberg M, Westpheling J (October 1991). "Streptomyces: a host for heterologous gene expression". Current Opinion in Biotechnology. 2 (5): 674–81. doi:10.1016/0958-1669(91)90033-2. PMID 1367716.
- ↑ Payne GF, DelaCruz N, Coppella SJ (July 1990). "Improved production of heterologous protein from Streptomyces lividans". Applied Microbiology and Biotechnology. 33 (4): 395–400. doi:10.1007/BF00176653. PMID 1369282. S2CID 19287805.
- 1 2 Binnie C, Cossar JD, Stewart DI (August 1997). "Heterologous biopharmaceutical protein expression in Streptomyces". Trends in Biotechnology. 15 (8): 315–20. doi:10.1016/S0167-7799(97)01062-7. PMID 9263479.
- ↑ Watve MG, Tickoo R, Jog MM, Bhole BD (November 2001). "How many antibiotics are produced by the genus Streptomyces?". Archives of Microbiology. 176 (5): 386–90. doi:10.1007/s002030100345. PMID 11702082. S2CID 603765.
- ↑ Procópio RE, Silva IR, Martins MK, Azevedo JL, Araújo JM (2012). "Antibiotics produced by Streptomyces". The Brazilian Journal of Infectious Diseases. 16 (5): 466–71. doi:10.1016/j.bjid.2012.08.014. PMID 22975171.
- ↑ Akagawa H, Okanishi M, Umezawa H (October 1975). "A plasmid involved in chloramphenicol production in Streptomyces venezuelae: evidence from genetic mapping". Journal of General Microbiology. 90 (2): 336–46. doi:10.1099/00221287-90-2-336. PMID 1194895.
- ↑ Miao V, Coëffet-LeGal MF, Brian P, Brost R, Penn J, Whiting A, et al. (May 2005). "Daptomycin biosynthesis in Streptomyces roseosporus: cloning and analysis of the gene cluster and revision of peptide stereochemistry". Microbiology. 151 (Pt 5): 1507–1523. doi:10.1099/mic.0.27757-0. PMID 15870461.
- ↑ Woodyer RD, Shao Z, Thomas PM, Kelleher NL, Blodgett JA, Metcalf WW, et al. (November 2006). "Heterologous production of fosfomycin and identification of the minimal biosynthetic gene cluster". Chemistry & Biology. 13 (11): 1171–82. doi:10.1016/j.chembiol.2006.09.007. PMID 17113999.
- ↑ Peschke U, Schmidt H, Zhang HZ, Piepersberg W (June 1995). "Molecular characterization of the lincomycin-production gene cluster of Streptomyces lincolnensis 78-11". Molecular Microbiology. 16 (6): 1137–56. doi:10.1111/j.1365-2958.1995.tb02338.x. PMID 8577249. S2CID 45162659.
- ↑ Dulmage HT (March 1953). "The production of neomycin by Streptomyces fradiae in synthetic media". Applied Microbiology. 1 (2): 103–6. doi:10.1128/AEM.1.2.103-106.1953. PMC 1056872. PMID 13031516.
- ↑ Sankaran L, Pogell BM (December 1975). "Biosynthesis of puromycin in Streptomyces alboniger: regulation and properties of O-demethylpuromycin O-methyltransferase". Antimicrobial Agents and Chemotherapy. 8 (6): 721–32. doi:10.1128/AAC.8.6.721. PMC 429454. PMID 1211926.
- ↑ Distler J, Ebert A, Mansouri K, Pissowotzki K, Stockmann M, Piepersberg W (October 1987). "Gene cluster for streptomycin biosynthesis in Streptomyces griseus: nucleotide sequence of three genes and analysis of transcriptional activity". Nucleic Acids Research. 15 (19): 8041–56. doi:10.1093/nar/15.19.8041. PMC 306325. PMID 3118332.
- ↑ Nelson M, Greenwald RA, Hillen W (2001). Tetracyclines in biology, chemistry and medicine. Birkhäuser. pp. 8–. ISBN 978-3-7643-6282-9. Retrieved 17 January 2012.
- ↑ "What are Streptomycetes?". Hosenkin Lab; Hiroshima-University. Archived from the original on 4 March 2016. Retrieved 10 August 2015.
- ↑ Swan DG, Rodríguez AM, Vilches C, Méndez C, Salas JA (February 1994). "Characterisation of a Streptomyces antibioticus gene encoding a type I polyketide synthase which has an unusual coding sequence". Molecular & General Genetics. 242 (3): 358–62. doi:10.1007/BF00280426. PMID 8107683. S2CID 2195072.
- ↑ "Finto: MeSH: Streptomyces antibioticus". finto: Finnish Thesaurus and Ontology Service. Retrieved 10 August 2015.
- ↑ Atta HM (January 2015). "Biochemical studies on antibiotic production from Streptomyces sp.: Taxonomy, fermentation, isolation and biological properties". Journal of Saudi Chemical Society. 19 (1): 12–22. doi:10.1016/j.jscs.2011.12.011.
- ↑ Oh DC, Scott JJ, Currie CR, Clardy J (February 2009). "Mycangimycin, a polyene peroxide from a mutualist Streptomyces sp". Organic Letters. 11 (3): 633–6. doi:10.1021/ol802709x. PMC 2640424. PMID 19125624.
- ↑ Chen TS, Chang CJ, Floss HG (June 1981). "Biosynthesis of boromycin". The Journal of Organic Chemistry. 46 (13): 2661–2665. doi:10.1021/jo00326a010.
- ↑ "CID=53385491". PubChem Compound Database. National Center for Biotechnology Information. Retrieved 8 March 2017.
- ↑ Holmes TC, May AE, Zaleta-Rivera K, Ruby JG, Skewes-Cox P, Fischbach MA, et al. (October 2012). "Molecular insights into the biosynthesis of guadinomine: a type III secretion system inhibitor". Journal of the American Chemical Society. 134 (42): 17797–806. doi:10.1021/ja308622d. PMC 3483642. PMID 23030602.
- ↑ Abe, N.; Nakakita, Y.; Nakamura, T.; Enoki, N.; Uchida, H.; Munekata, M. (1993). "Novel antitumor antibiotics, saptomycins. I. Taxonomy of the producing organism, fermentation, HPLC analysis and biological activities". The Journal of Antibiotics. 46 (10): 1530–5. doi:10.7164/antibiotics.46.1530. PMID 8244880.
Further reading
- Baumberg S (1991). Genetics and Product Formation in Streptomyces. Kluwer Academic. ISBN 978-0-306-43885-1.
- Gunsalus IC (1986). Bacteria: Antibiotic-producing Streptomyces. Academic Press. ISBN 978-0-12-307209-2.
- Hopwood DA (2007). Streptomyces in Nature and Medicine: The Antibiotic Makers. Oxford University Press. ISBN 978-0-19-515066-7.
- Dyson P, ed. (2011). Streptomyces: Molecular Biology and Biotechnology. Caister Academic Press. ISBN 978-1-904455-77-6.
External links
- "Current research on Streptomyces coelicolor". Norwich Research Park. 3 January 2018.
- "Some current Streptomyces Research & Methods / Protocols / Resources". www.openwetware.org.
- "S. avermitilis genome homepage". Kitasato Institute for Life Sciences.
- "S. coelicolor A3(2) genome homepage". Sanger Institute.
- "Streptomyces.org.uk homepage". John Innes Centre.
- "StrepDB - the Streptomyces genomes annotation browser".
- "Streptomyces Genome Projects". Genomes OnLine Database.