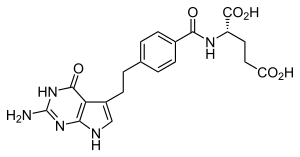
Pemetrexed
 | |
 | |
| Names | |
|---|---|
| Trade names | Alimta, Pemfexy, Ciambra, others |
IUPAC name
| |
| Clinical data | |
| Main uses | Mesothelioma, non-small cell lung cancer (NSCLC)[1] |
| Side effects | Fever, infection, inflammation of the mouth, rash, nausea, tiredness, shortness of breath, chest pain[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Intravenous |
| Typical dose | 500 mg/m2[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | NA |
| Protein binding | 81% |
| Metabolism | Negligible |
| Elimination half-life | 3.5 hours |
| Excretion | Kidney |
| Chemical and physical data | |
| Formula | C20H21N5O6 |
| Molar mass | 427.417 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Pemetrexed, sold under the brand name Alimta among others, is a medication used to treat mesothelioma and non-small cell lung cancer (NSCLC).[1] It is used for mesothelioma which involves the pleura and cannot be removed by surgery.[2] It is given by gradual injection into a vein.[1]
Common side effects include fever, infection, inflammation of the mouth, rash, nausea, tiredness, shortness of breath, and chest pain.[2] Other side effects may include bone marrow suppression.[2] Use in pregnancy may harm the baby.[2] It works by blocking the creation of DNA and RNA by inhibiting thymidylate transferase.[4][1]
Pemetrexed was approved for medical use in the United States and Europe in 2004.[4][2] It is available as a generic medication.[1] In the United Kingdom 500 mg costs the NHS about £450 as of 2021.[1] This amount in the United States is about 3,700 USD.[5]
Medical use
It is used for the treatment of malignant pleural mesothelioma, a type of tumor of the mesothelium, the thin layer of tissue that covers many of the internal organs, in combination with cisplatin[6] in people whose disease is either unresectable or who are not otherwise candidates for curative surgery.[7] It is a first-line treatment, in combination with cisplatin, against locally advanced and metastatic non-small cell lung cancer (NSCLC) in people with non-squamous histology.[8][9][3]
Carboplatin
Pemetrexed is also recommended in combination with carboplatin and pembrolizumab for the first-line treatment of advanced non-small cell lung cancer.[10][11] However, the relative efficacy or toxicity of pemetrexed-cisplatin versus pemetrexed-carboplatin has not been established beyond what is generally thought about cisplatin or carboplatin doublet drug therapy.[12]
Supplementation
Patients are recommended to take folic acid and vitamin B12 supplement even if levels are normal when they are on pemetrexed therapy.[13][3] (In clinical trials for mesothelioma, folic acid and B12 supplementation reduced the frequency of adverse events.) It is also recommended for patients to be on a glucocorticoid (e.g. dexamethasone) on the day prior, day of, and day after pemetrexed infusion to avoid skin rashes.[3]
Dosage
It is given at a dose of 500 mg/m2 every three weeks.[2]
Side effects
Pemetrexed, whether used alone or in combination with cisplatin, has these side effects:[3]
- Low blood cell counts, as measured by a complete blood count. This is a dose-limiting toxicity.
- Mental fatigue and sleepiness. Fatigue can be reduced through an off-label prescription of modafinil.[14]
- Nausea and vomiting. Pemetrexed's emetogenic effects are managed with prophylactic antiemetics.
- Diarrhea
- Oral mucositis (mouth, throat, or lip sores). Oral ulcers can be mitigated by proper oral hygiene, including rinsing of the mouth with salt water following consumption of food or drink.[15]
- Loss of appetite
- Skin rash. Physician-prescribed glucocorticoids administered on the day prior, day of, and day after infusion typically avoid skin rashes.
- Constipation
Mechanism of action
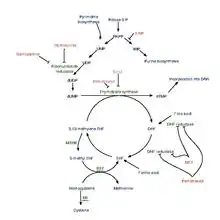
Pemetrexed is chemically similar to folic acid and is in the class of chemotherapy drugs called folate antimetabolites. It works by inhibiting three enzymes used in purine and pyrimidine synthesis—thymidylate synthase (TS), dihydrofolate reductase (DHFR), and glycinamide ribonucleotide formyltransferase[16][17] (GARFT). By inhibiting the formation of precursor purine and pyrimidine nucleotides, pemetrexed prevents the formation of DNA and RNA, which are required for the growth and survival of both normal cells and cancer cells.
History
The molecular structure of pemetrexed was developed by Edward C. Taylor at Princeton University and clinically developed by Indianapolis-based drug maker, Eli Lilly and Company in 2004.
It has been researched in the PARAMOUNT trial.
Society and culture
Trade names
In addition to the brand name Alimta, this drug is also marketed in India by Abbott Healthcare as Pleumet and by Cadila Healthcare as Pemecad. In February 2020, Pemfexy was approved for use in the United States.[18]
Cost
In the United States, as of 2015, each vial of medicine costs between US$2,623 and US$3,100.[19]
Research
A Phase III study showed benefits of maintenance use of pemetrexed for non-squamous NSCLC.[20] Activity has been shown in malignant peritoneal mesothelioma.[21] Trials are currently testing it against esophageal cancer, chordoma[22] and other cancers.
References
- 1 2 3 4 5 6 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 960. ISBN 978-0857114105.
- 1 2 3 4 5 6 7 8 "PEMEtrexed Monograph for Professionals". Drugs.com. Archived from the original on 20 April 2019. Retrieved 27 October 2021.
- 1 2 3 4 5 "Alimta- pemetrexed disodium injection, powder, lyophilized, for solution". DailyMed. 25 March 2020. Archived from the original on 20 October 2020. Retrieved 20 October 2020.
- 1 2 "Alimta". Archived from the original on 18 January 2021. Retrieved 27 October 2021.
- ↑ "Pemetrexed Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 27 October 2021.
- ↑ Manegold C (August 2003). "Pemetrexed (Alimta, MTA, multitargeted antifolate, LY231514) for malignant pleural mesothelioma". Semin. Oncol. 30 (4 Suppl 10): 32–6. doi:10.1016/S0093-7754(03)00283-5. PMID 12917819.
- ↑ National Cancer Institute: FDA Approval for Pemetrexed Disodium Archived 6 April 2015 at the Wayback Machine
- ↑ Cohen MH, Justice R, Pazdur R (September 2009). "Approval summary: pemetrexed in the initial treatment of advanced/metastatic non-small cell lung cancer". Oncologist. 14 (9): 930–5. doi:10.1634/theoncologist.2009-0092. PMID 19737998.
- ↑ Rossi A, Ricciardi S, Maione P, de Marinis F, Gridelli C (November 2009). "Pemetrexed in the treatment of advanced non-squamous lung cancer". Lung Cancer. 66 (2): 141–9. doi:10.1016/j.lungcan.2009.06.006. PMID 19577816.
- ↑ Ettinger DS et al. NCCN Clinical Practice Guidelines in Oncology: Non-small Cell Lung Cancer V.2.2009 available from www.nccn.org
- ↑ Gandhi, Leena; Rodríguez-Abreu, Delvys; Gadgeel, Shirish; Esteban, Emilio; Felip, Enriqueta; De Angelis, Flávia; Domine, Manuel; Clingan, Philip; Hochmair, Maximilian J.; Powell, Steven F.; Cheng, Susanna Y.-S.; Bischoff, Helge G.; Peled, Nir; Grossi, Francesco; Jennens, Ross R.; Reck, Martin; Hui, Rina; Garon, Edward B.; Boyer, Michael; Rubio-Viqueira, Belén; Novello, Silvia; Kurata, Takayasu; Gray, Jhanelle E.; Vida, John; Wei, Ziwen; Yang, Jing; Raftopoulos, Harry; Pietanza, M. Catherine; Garassino, Marina C.; KEYNOTE-189 Investigators (2018). "Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer". New England Journal of Medicine. 378 (22): 2078–2092. doi:10.1056/NEJMoa1801005. hdl:10138/298862. PMID 29658856.
- ↑ Azzoli CG, Kris MG, Pfister DG (June 2007). "Cisplatin versus carboplatin for patients with metastatic non-small-cell lung cancer—an old rivalry renewed". J. Natl. Cancer Inst. 99 (11): 828–9. doi:10.1093/jnci/djk222. PMID 17551137.
- ↑ Hazarika M, White RM, Johnson JR, Pazdur R (2004). "FDA drug approval summaries: pemetrexed (Alimta)". Oncologist. 9 (5): 482–8. doi:10.1634/theoncologist.9-5-482. PMID 15477632.
- ↑ James P.; Wilmot Cancer Center. "Scientists Help Breast Cancer Survivor Lift the Fog of "Chemo Brain"" (PDF). Dialogue. University of Rochester Medical Center. Fall 2007: 2–3. Retrieved 1 December 2008.
- ↑ "Oral Complications of Chemotherapy and Head/Neck Radiation". Cancer Topics – Coping with Cancer. National Cancer Institute. Archived from the original on 6 December 2008. Retrieved 1 December 2008.
- ↑ McLeod HL, Cassidy J, Powrie RH, Priest DG, Zorbas MA, Synold TW, Shibata S, Spicer D, Bissett D, Pithavala YK, Collier MA, Paradiso LJ, Roberts JD (July 2000). "Pharmacokinetic and Pharmacodynamic Evaluation of the Glycinamide Ribonucleotide Formyltransferase Inhibitor AG2034". Clinical Cancer Research; Clinical Trials. 6 (7): 2677–84. PMID 10914709. Archived from the original on 28 July 2009. Retrieved 7 May 2021.
- ↑ Avendano, Carmen; Menendez, J. Carlos (April 2008). Medicinal Chemistry of Anticancer Drugs. Amsterdam: Elsevier. p. 37. ISBN 978-0-444-52824-7.
- ↑ "Pemfexy: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 19 October 2020. Retrieved 13 February 2020.
- ↑ Langreth, Robert (29 June 2016). "Decoding Big Pharma's Secret Drug Pricing Practices". Bloomberg. Archived from the original on 13 July 2016. Retrieved 15 July 2016.
- ↑ "Maintenance pemetrexed (Pem) plus best supportive care (BSC) versus placebo (Plac) plus BSC: A randomized phase III study in advanced non-small cell lung cancer (NSCLC)". American Society of Clinical Oncology. Archived from the original on 16 June 2009. Retrieved 22 July 2009.
- ↑ Carteni G, Manegold C, Garcia GM, et al. (May 2009). "Malignant peritoneal mesothelioma-Results from the International Expanded Access Program using pemetrexed alone or in combination with a platinum agent". Lung Cancer. 64 (2): 211–8. doi:10.1016/j.lungcan.2008.08.013. PMID 19042053.
- ↑ "Archive copy". Archived from the original on 25 April 2021. Retrieved 7 May 2021.
{{cite web}}: CS1 maint: archived copy as title (link)
External links
| Identifiers: |
|---|
- "Pemetrexed". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 19 May 2021. Retrieved 7 May 2021.
- "Pemetrexed disodium". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 22 October 2020. Retrieved 7 May 2021.
- "Pemetrexed disodium". NCI Drug Dictionary. National Cancer Institute. Archived from the original on 23 October 2020. Retrieved 7 May 2021.
- "Pemetrexed disodium". National Cancer Institute. Archived from the original on 9 April 2021. Retrieved 7 May 2021.