Verteporfin
 | |
| Names | |
|---|---|
| Trade names | Visudyne, others |
IUPAC name
| |
| Clinical data | |
| Main uses | Wet form of age-related macular degeneration, choroidal neovascularisation due to severe short-sightedness[1] |
| Side effects | Pain at the site of injection, vision changes[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Intravenous |
| Typical dose | 6 mg/m2[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607060 |
| Legal | |
| License data | |
| Legal status |
|
| Chemical and physical data | |
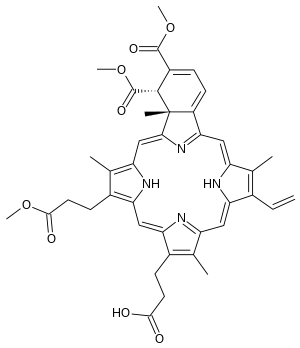
| Formula | C41H42N4O8 |
| Molar mass | 718.807 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Verteporfin, sold under the trade name Visudyne among others, is a medication used to treat the wet form of age-related macular degeneration and choroidal neovascularisation due to severe short-sightedness.[1] It is given by injection into a vein followed by the use of a laser to activate it.[1][3] Both eyes may be treated following a single dose.[1]
Common side effects include pain at the site of injection and vision changes.[2] Other side effects may include shortness of breath, headache, tiredness, and fever.[3] It should not be used in those with acute porphyria.[3] It is a benzoporphyrin derivative.[2] Once activated it creates oxygen molecules in the area which block off excessive blood vessels.[1]
Verteporfin was approved for medical use in the Europe and the United States in 2000.[2][1] In the United Kingdom a vial of 15 mg costs the NHS about £850 as of 2021.[3] In the United States this amount costs about 1,800 USD.[4]
Medical use
Administration
Verteporfin is given intravenously, 15 minutes before laser treatment.[5]
It is given at a dose of 6 mg/m2.[3]
Contraindications
Side effects
Most common side effects are blurred vision, headache, and local effects at the injection site. Also, photosensitivity; it is strictly advised to avoid exposure to sunlight and unscreened lighting until 48 hours after verteporfin administration.[5]
Interactions
Verteporfin is known to interact with the herbal remedy feverfew (Tanacetum parthenium), the latter of which seems to act as an antagonist to verteporfin for unknown reasons. Taking the two substances simultaneously is inadvisable.[6]
Verteporfin does not appear to be metabolized by Cytochrome P450 enzymes, therefore not affecting Cytochrome P450 metabolism of other drugs.[7]
Research
Verteporfin is an inhibitor of fibrosis in patients with persistent cholestasis.[8] Verteporfin prevents fibrosis in several human organs.[8] In 2018 information revealed verteporfin stopped fibrosis in the lung.[8] Verteporfin is a marketed drug with a good safety profile. Verteporfin has also been used off-label.[9]
In 2021, scientists tested verteporfin to reveal if the drug would prevent scar tissue in skin.[10] Testing of verteporfin on humans cleft lips will occur in 2021.[11]
References
- 1 2 3 4 5 6 "Visudyne". Archived from the original on 27 May 2021. Retrieved 13 September 2021.
- 1 2 3 4 "Verteporfin Monograph for Professionals". Drugs.com. Archived from the original on 22 May 2021. Retrieved 13 September 2021.
- 1 2 3 4 5 6 BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 1256. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ↑ "Visudyne Prices, Coupons & Patient Assistance Programs". Drugs.com. Retrieved 13 September 2021.
- 1 2 3 Verteporfin Monograph
- ↑ "Feverfew and Verteporfin Interactions". Drugs.com. Archived from the original on 14 April 2015. Retrieved 14 April 2015.
- ↑ "Visudyne (verteporfin for injection) prescribing information" (PDF). FDA. FDA. Archived (PDF) from the original on 30 March 2021. Retrieved 12 April 2021.
- 1 2 3 Clark, Richard A. F. (29 July 2021), "To Scar or Not to Scar", N Engl J Med, doi:10.1056/NEJMcibr2107204, archived from the original on 30 July 2021, retrieved 3 August 2021
- ↑ Karim SP, Adelman RA (2013). "Profile of verteporfin and its potential for the treatment of central serous chorioretinopathy". Clinical Ophthalmology. 7: 1867–75. doi:10.2147/OPTH.S32177. PMC 3788817. PMID 24092965.
- ↑ Molteni, Megan, In mouse experiments, scientists unlock the key to scar-free skin healing Archived 2021-04-30 at the Wayback Machine, STAT News, April 22, 2021
- ↑ Kolata, Gina (22 April 2021). "Imagine, Surgery Without a Scar". nytimes,com. nytimes,com. Archived from the original on 3 August 2021. Retrieved 3 August 2021.
External links
| Identifiers: |
|---|
- "Visudyne". Novartis. Archived from the original on 2014-09-03. Retrieved 2021-08-03.