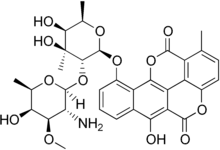
Elsamitrucin
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C33H35NO13 |
| Molar mass | 653.637 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Elsamitrucin (elsamicin A) is a drug used in chemotherapy.[1] Elsamitrucin is chemically related to chartreusin.
References
- ↑ Barceló F, Portugal J (October 2004). "Elsamicin A binding to DNA. A comparative thermodynamic characterization". FEBS Letters. 576 (1–2): 68–72. doi:10.1016/j.febslet.2004.08.063. PMID 15474012.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.