Daunorubicin
 | |
 | |
| Names | |
|---|---|
| Trade names | Cerubidine, others |
IUPAC name
| |
| Clinical data | |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Exclusively intravenous. Causes severe necrosis if administered intramuscularly or subcutaneously |
| Defined daily dose | not established[1] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682289 |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Metabolism | Liver |
| Elimination half-life | 26.7 hours (metabolite) |
| Excretion | Biliary and urinary |
| Chemical and physical data | |
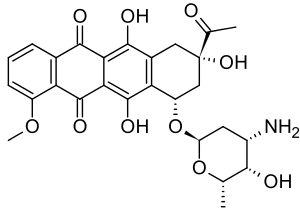
| Formula | C27H29NO10 |
| Molar mass | 527.52 g/mol 563.99 g/mol (HCl salt) g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Daunorubicin, also known as daunomycin, is a chemotherapy medication used to treat cancer.[2] Specifically it is used for acute myeloid leukemia (AML), acute lymphocytic leukemia (ALL), chronic myelogenous leukemia (CML), and Kaposi's sarcoma.[2] It is used by injection into a vein.[2] A liposomal formulation known as liposomal daunorubicin also exists.[2]
Common side effects include hair loss, vomiting, bone marrow suppression, and inflammation of the inside of the mouth.[2] Other severe side effects include heart disease and tissue death at the site of injection.[2] Use in pregnancy may harm the baby.[2] Daunorubicin is in the anthracycline family of medication.[3] It works in part by blocking the function of topoisomerase II.[2]
Daunorubicin was approved for medical use in the United States in 1979.[2] It is on the World Health Organization's List of Essential Medicines.[4] The wholesale cost in the developing world is about 5.79 to 37.18 USD for a 20 mg vial.[5] This amount in the United Kingdom costs the NHS about 55.00 pounds.[3] It was originally isolated from bacteria of the Streptomyces type.[6]
Medical uses
It slows or stops the growth of cancer cells in the body. Treatment is usually performed together with other chemotherapy drugs (such as cytarabine), and its administration depends on the type of tumor and the degree of response.
In addition to its major use in treating AML, daunorubicin is also used to treat neuroblastoma. Daunorubicin has been used with other chemotherapy agents to treat the blastic phase of chronic myelogenous leukemia.
Daunorubicin is also used as the starting material for semi-synthetic manufacturing of doxorubicin, epirubicin and idarubicin.
Dosage
The defined daily dose is not established[1]
Mechanism of action
Similar to doxorubicin, daunorubicin interacts with DNA by intercalation and inhibition of macromolecular biosynthesis.[7][8] This inhibits the progression of the enzyme topoisomerase II, which relaxes supercoils in DNA for transcription. Daunorubicin stabilizes the topoisomerase II complex after it has broken the DNA chain for replication, preventing the DNA double helix from being resealed and thereby stopping the process of replication. On binding to DNA, daunomycin intercalates, with its daunosamine residue directed toward the minor groove. It has the highest preference for two adjacent G/C base pairs flanked on the 5' side by an A/T base pair. Crystallography shows that daunomycin induces a local unwinding angle of 8°, and other conformational disturbances of adjacent and second-neighbour base pairs.[9] It can also induce histone eviction from chromatin upon intercalation.[10][11]
History
In the 1950s, an Italian research company, Farmitalia Research Laboratories, began an organized effort to isolate anticancer compounds from soil-based microbes. A soil sample was isolated from the area surrounding the Castel del Monte, a 13th-century castle in Apulia. A new strain of Streptomyces peucetius which produced a red pigment was isolated, and an antibiotic was produced from this bacterium that was found to have good activity against murine tumors. Since a group of French researchers discovered the same compound at about the same time, the two teams named the compound daunorubicin, combining the name Dauni, a pre-Roman tribe that occupied the area of Italy where the compound was isolated, with the French word for ruby, rubis, describing the color.[12][13][14] Clinical trials began in the 1960s, and the drug saw success in treating acute leukemia and lymphoma.
However, by 1967, it was recognized that daunorubicin could produce fatal cardiac toxicity.[15]
In 2015–16, a team at Ohio State University "showed that, by carefully manipulating strands of viral DNA, an origami structure with complex folds can be created in just 10 minutes. Incredibly, these structures are only 100 nanometers across – that’s 1,000 times smaller than the width of a human hair. Small volumes of daunorubicin can be wrapped up in these minuscule pods, which can then be released into a leukemia cell-filled environment."[16]
Route of administration
Daunorubicin should only be administered in a rapid intravenous infusion. It should not be administered intramuscularly or subcutaneously, since it may cause extensive tissue necrosis. It should also never be administered intrathecally (into the spinal canal), as this will cause extensive damage to the nervous system and may lead to death. Daunorubicin has been used intravitreally (inside the eye) for the purposes of preventing proliferative vitreoretinopathy, a common complication following retinal detachment surgery, but has not been found to be effective and is not used for any other ophthalmic purposes at this time.[17]
See also
References
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 28 November 2020. Retrieved 17 September 2020.
- 1 2 3 4 5 6 7 8 9 "daunorubicin hydrochloride". The American Society of Health-System Pharmacists. Archived from the original on 8 January 2017. Retrieved 8 December 2016.
- 1 2 British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. pp. 581–583. ISBN 9780857111562.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "Daunorubicin". International Drug Price Indicator Guide. Archived from the original on 9 October 2018. Retrieved 8 December 2016.
- ↑ Lin, Guo-Qiang; You, Qi-Dong; Cheng, Jie-Fei (2011). Chiral Drugs: Chemistry and Biological Action. John Wiley & Sons. p. 120. ISBN 9781118075630. Archived from the original on 2016-12-21.
- ↑ Fornari FA, Randolph JK, Yalowich JC, Ritke MK, Gewirtz DA (April 1994). "Interference by doxorubicin with DNA unwinding in MCF-7 breast tumor cells". Mol Pharmacol. 45 (4): 649–56. PMID 8183243.
- ↑ Momparler RL, Karon M, Siegel SE, Avila F (August 1976). "Effect of adriamycin on DNA, RNA, and protein synthesis in cell-free systems and intact cells". Cancer Res. 36 (8): 2891–5. PMID 1277199. Archived from the original on 2009-02-05.
- ↑ G J Quigley; A H Wang; G Ughetto; G van der Marel; J H van Boom; A Rich (December 1980). "Molecular structure of an anticancer drug-DNA complex: daunomycin plus d(CpGpTpApCpG)". PNAS. 77 (12): 7204–7208. doi:10.1073/pnas.77.12.7204. PMC 350470. PMID 6938965.
{{cite journal}}: Unknown parameter|last-author-amp=ignored (help) - ↑ Pang B, Qiao X, Janssen L, Velds A, Groothuis T, Kerkhoven R, Nieuwland M, Ovaa H, Rottenberg S, van Tellingen O, Janssen J, Huijgens P, Zwart W, Neefjes J (2013). "Drug-induced histone eviction from open chromatin contributes to the chemotherapeutic effects of doxorubicin". Nature Communications. 4: 1908. doi:10.1038/ncomms2921. PMC 3674280. PMID 23715267.
- ↑ Pang B, de Jong J, Qiao X, Wessels LF, Neefjes J (2015). "Chemical profiling of the genome with anti-cancer drugs defines target specificities". Nature Chemical Biology. 11 (7): 472–80. doi:10.1038/nchembio.1811. PMID 25961671.
- ↑ Weiss RB (December 1992). "The anthracyclines: will we ever find a better doxorubicin?". Seminars in Oncology. 19 (6): 670–86. PMID 1462166.
- ↑ Baruffa G (1966). "Clinical trials in Plasmodium falciparum malaria with a long-acting sulphonamide". Trans. R. Soc. Trop. Med. Hyg. 60 (2): 222–4. doi:10.1016/0035-9203(66)90030-7. PMID 5332105.
- ↑ Per prior citation, the first publication: Camerino B, Palamidessi G (1960) Derivati della parazina II. Sulfonamdopir (in Italian). Gazz Chim Ital 90:1802–1815
- ↑ Tan C, Tasaka H, Yu KP, Murphy ML, Karnofsky DA (March 1967). "Daunomycin, an antitumor antibiotic, in the treatment of neoplastic disease. Clinical evaluation with special reference to childhood leukemia". Cancer. 20 (3): 333–53. doi:10.1002/1097-0142(1967)20:3<333::AID-CNCR2820200302>3.0.CO;2-K. PMID 4290058.
- ↑ "http://www.iflscience.com/health-and-medicine/researchers-kill-drug-resistant-leukemia-cells-using-dna-trojan-horse-attack/". IFL Science. Archived from the original on 2016-02-29. Retrieved 2016-02-27.
{{cite web}}: External link in|title= - ↑ Mortensen, ME; et al. (1992). "Inadvertent intrathecal injection of daunorubicin with fatal outcome". Med Pediatr Oncol. 20 (3): 249–253. doi:10.1002/mpo.2950200315. PMID 1574039.
External links
| External sites: |
|
|---|---|
| Identifiers: |