Losoxantrone
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
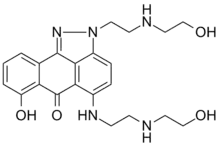
| Formula | C22H27N5O4 |
| Molar mass | 425.489 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Losoxantrone (biantrazole) is an anthroquinone anthrapyrazole antineoplastic agent and analog of mitoxantrone.[1][2] It is also sometimes known as DuP 941.[3]
See also
- Mitoxantrone
- Piroxantrone
References
- ↑ Showalter HD, Johnson JL, Hoftiezer JM, Turner WR, Werbel LM, Leopold WR, et al. (January 1987). "Anthrapyrazole anticancer agents. Synthesis and structure-activity relationships against murine leukemias". Journal of Medicinal Chemistry. 30 (1): 121–31. doi:10.1021/jm00384a021. PMID 3806589.
- ↑ Beylin VG, Colbry NL, Goel OP, Haky JE, Johnson DR, Johnson JL, Kanter GD, Leeds RL, Leja B, Lewis EP, Rithner CD (1989). "Anticancer anthrapyrazoles. Improved syntheses of clinical agents CI-937, CI-941, and piroxantrone hydrochloride". Journal of Heterocyclic Chemistry. 26: 85–96. doi:10.1002/jhet.5570260117.
- ↑ Leteurtre F, Kohlhagen G, Paull KD, Pommier Y (August 1994). "Topoisomerase II inhibition and cytotoxicity of the anthrapyrazoles DuP 937 and DuP 941 (Losoxantrone) in the National Cancer Institute preclinical antitumor drug discovery screen". Journal of the National Cancer Institute. 86 (16): 1239–44. doi:10.1093/jnci/86.16.1239. PMID 8040892.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.