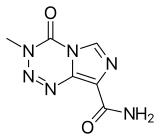
Temozolomide
 | |
 | |
| Names | |
|---|---|
| Trade names | Temodar, Temodal, Temcad, others[1] |
IUPAC name
| |
| Clinical data | |
| Drug class | Alkylating agent[2] |
| Main uses | Glioblastoma, anaplastic astrocytoma[2] |
| Side effects | Nausea, vomiting, constipation, loss of appetite, hair loss, headache, tiredness, seizures, rash, low white blood cells, low platelet[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | By mouth, intravenous |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601250 |
| Legal | |
| License data |
|
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | almost 100% |
| Protein binding | 15% (10–20%) |
| Metabolism | hydrolysis |
| Metabolites | 3-methyl-(triazen-1-yl)imidazole-4-carboxamide (MTIC, the active species); temozolomide acid |
| Elimination half-life | 1.8 hours |
| Excretion | mainly kidney |
| Chemical and physical data | |
| Formula | C6H6N6O2 |
| Molar mass | 194.154 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 212 °C (414 °F) (decomp.) |
SMILES
| |
InChI
| |
Temozolomide (TMZ), sold under the brand name Temodar among others, is a medication used to treat brain tumors such as glioblastoma and anaplastic astrocytoma.[2] It is taken by mouth or by gradual injection into a vein.[2]
Common side include nausea, vomiting, constipation, loss of appetite, hair loss, headache, tiredness, seizures, rash, low white blood cells, and low platelet.[2] People receiving the injection may also have pain, irritation, itching, redness, or bruising at the site.[2] Other side effects may include Pneumocystis jiroveci pneumonia, other cancers, and hepatitis B reactivation.[4] It is an alkylating agent.[2]
Temozolomide was approved for medical use in the United States and Europe in 1999.[4][2] It is available as a generic medication.[5] In the United Kingdom five pills of 250 mg costs the NHS about £530 as of 2021.[5] This amount in the United States costs about 135 USD.[6]
Medical uses
In the United States temozolomide is indicated for the treatment of adults with newly diagnosed glioblastoma concomitantly with radiotherapy and subsequently as monotherapy treatment;[7] or adults with refractory anaplastic astrocytoma who have experienced disease progression on a drug regimen containing nitrosourea and procarbazine.[7]
In the European Union temozolomide is indicated for adults with newly diagnosed glioblastoma concomitantly with radiotherapy and subsequently as monotherapy treatment;[2][8] or children from the age of three years, adolescents and adults with malignant glioma, such as glioblastoma multiforme or anaplastic astrocytoma, showing recurrence or progression after standard therapy.[2][8]
Olaparib in combination with temozolomide may also be useful small cell lung cancer.[9]
Dosage
It is generally given at a dose of 75 to 200 mg per m2 of body surface area.[2]
Contraindications
Temozolomide is contraindicated in people with hypersensitivity to it or to the similar drug dacarbazine. The use of temozolomide is not recommended in people with severe myelosuppression.[10]
Side effects
The most common side effect is bone marrow suppression. The most common non-hematological adverse effects associated with temozolomide are nausea and vomiting, which are either self-limiting or readily controlled with standard antiemetic therapy. These latter effects are usually mild to moderate (grade 1 to 2). The incidence of severe nausea and vomiting is around 4% each. Patients who have pre-existing or a history of severe vomiting may require antiemetic therapy before initiating temozolomide treatment. Temozolomide should be administered in the fasting state, at least one hour before a meal. Antiemetic therapy may be administered before, or following, administration of temozolomide.
Temozolomide is genotoxic, teratogenic and fetotoxic and should not be used during pregnancy. Lactating women should discontinue nursing while receiving the drug because of the risk of secretion into breast milk. One study indicated that women that have taken temozolomide without concomitant fertility preservation measures achieve pregnancy to a lesser rate later in life, but the study was too small to show statistical significance in the hypothesis that temozolomide would confer a risk of female infertility.[11] In male patients, temozolomide can have genotoxic effects. Men are advised not to father a child during or up to six months after treatment and to seek advice on cryoconservation of sperm prior to treatment, because of the possibility of irreversible infertility due to temozolomide therapy.
Very rarely temozolomide can cause acute respiratory failure or liver damage.
Interactions
As temozolomide is not metabolized in the liver and has a low affinity to plasma proteins, it is expected to have a low potential for interactions. An analysis of patient data showed no interactions with a range of other drugs; the exception is valproic acid, which slightly slows down temozolomide elimination from the body. Combining the drug with other myelosuppressants may increase the risk of myelosuppression.[10]
Pharmacology
Mechanism of action
The therapeutic benefit of temozolomide depends on its ability to alkylate/methylate DNA, which most often occurs at the N-7 or O-6 positions of guanine residues. This methylation damages the DNA and triggers the death of tumor cells. However, some tumor cells are able to repair this type of DNA damage, and therefore diminish the therapeutic efficacy of temozolomide, by expressing a protein O6-alkylguanine DNA alkyltransferase (AGT) encoded in humans by the O-6-methylguanine-DNA methyltransferase (MGMT) gene.[12] In some tumors, epigenetic silencing of the MGMT gene prevents the synthesis of this enzyme, and as a consequence such tumors are more sensitive to killing by temozolomide.[13] Conversely, the presence of AGT protein in brain tumors predicts poor response to temozolomide and these patients receive little benefit from chemotherapy with temozolomide.[14]
Pharmacokinetics
Temozolomide is quickly and almost completely absorbed from the gut, and readily penetrates the blood–brain barrier; the concentration in the cerebrospinal fluid is 30% of the concentration in the blood plasma. Intake with food decreases maximal plasma concentrations by 33% and the area under the curve by 9%. Only 15% (10–20%) of the substance are bound to blood plasma proteins. Temozolomide is a prodrug; it is spontaneously hydrolyzed at physiological pH to 3-methyl-(triazen-1-yl)imidazole-4-carboxamide (MTIC). MTIC splits into monomethylhydrazine, probably the active methylating agent, and 5-aminoimidazole-4-carboxamide (AIC). Other metabolites include temozolomide acid and unidentified hydrophilic substances.[10]
Plasma half-life is 1.8 hours. The substance and its metabolites are mainly excreted via the urine.[10]
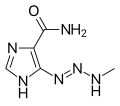 MTIC, the active metabolite
MTIC, the active metabolite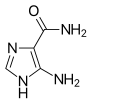 AIC (part of the naturally occurring AICA ribonucleotide)
AIC (part of the naturally occurring AICA ribonucleotide)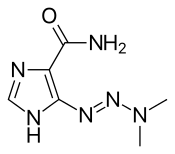 The related drug dacarbazine[15] for comparison
The related drug dacarbazine[15] for comparison
Chemical properties
Temozolomide is an imidazotetrazine derivative.[15] It is slightly soluble in water and aqueous acids,[16] and decomposes at 212 °C (414 °F).[17] It was recently discovered that temozolomide is an explosive, tentatively assigned as a Class 1 Explosive.[18][19]
History
The agent was developed by Malcolm Stevens and his team at Aston University in Birmingham, England.[15][20][21]
It was approved for medical use in the European Union in January 1999,[2] and in the United States in August 1999.[22] The intravenous formulation was approved in the United States in February 2009.[23]
Research
Laboratory studies and clinical trials have started investigating the possibility of increasing the anticancer potency of temozolomide by combining it with other pharmacologic agents. For example, clinical trials have indicated that the addition of chloroquine might be beneficial for the treatment of glioma patients.[24] Laboratory studies found that temozolomide killed brain tumor cells more efficiently when epigallocatechin gallate (EGCG), a component of green tea, was added; however, the efficacy of this effect has not yet been confirmed in brain-tumor patients.[25] Preclinical studies reported in 2010 on investigations into the use of the novel oxygen diffusion-enhancing compound trans sodium crocetinate (TSC) when combined with temozolomide and radiation therapy[26] and a clinical trial was underway as of August 2015.[27]
While the above-mentioned approaches have investigated whether the combination of temozolomide with other agents might improve therapeutic outcome, efforts have also started to study whether altering the temozolomide molecule itself can increase its activity. One such approach permanently fused perillyl alcohol, a natural compound with demonstrated therapeutic activity in brain cancer patients,[28] to the temozolomide molecule. The resultant novel compound, called NEO212 or TMZ-POH, revealed anticancer activity that was significantly greater than that of either of its two parent molecules, temozolomide and perillyl alcohol. Although as of 2016, NEO212 has not been tested in humans, it has shown superior cancer therapeutic activity in animal models of glioma,[29] melanoma,[30] and brain metastasis of triple-negative breast cancer.[31]
Because tumor cells that express the MGMT gene are more resistant to the effects of temozolomide, researchers investigated whether the inclusion of O6-benzylguanine (O6-BG), an AGT inhibitor, could overcome this resistance and improve the drug's therapeutic effectiveness. In the laboratory, this combination indeed showed increased temozolomide activity in tumor-cell culture in vitro and in animal models in vivo.[32] However, a recently completed phase-II clinical trial with brain-tumor patients yielded mixed outcomes; while there was some improved therapeutic activity when O6-BG and temozolomide were given to patients with temozolomide-resistant anaplastic glioma, there seemed to be no significant restoration of temozolomide sensitivity in patients with temozolomide-resistant glioblastoma multiforme.[33]
Some efforts focus on engineering hematopoietic stem cells expressing the MGMT gene prior to transplanting them into brain-tumor patients. This would allow for the patients to receive stronger doses of temozolomide, since the patient's hematopoietic cells would be resistant to the drug.[34]
High doses of temozolomide in high-grade gliomas have low toxicity, but the results are comparable to the standard doses.[35]
Two mechanisms of resistance to temozolomide effects have now been described: 1) intrinsic resistance conferred by MGMT deficiency (MGMTd) and 2) intrinsic or acquired resistance through MMR deficiency (MMRd). The MGMT enzyme is the first line of repair of mismatched bases created by TMZ. Cells are normally MGMT proficient (MGMTp) as they have an unmethylated MGMT promoter allowing the gene to be expressed normally. In this state, TMZ induced DNA damage is able to be efficiently repaired in tumor cells (and normal cells) by the active MGMT enzyme. Cells may grow and pass through the cell cycle normally without arrest or death. However, in some tumors cells are MGMT deficient (MGMTd). This is most commonly due to abnormal methylation of the MGMT gene promoter and suppression of gene expression. MGMTd has also been described to occur by promoter rearrangement. In cells with MGMTd, DNA damage by TMZ activates the next stage of repair in cells with a proficient Mismatch Repair enzyme complex (MMRp). In MMRp the MMR protein complex identifies the damage and causes cells to arrest and undergo death which inhibits tumor growth. However, if cells have combined MGMTd and MMR deficiency (MGMTd + MMRd) then cells retain the induced mutations and continue to cycle and are resistant to effects of TMZ.
In gliomas and other cancers MMRd has now been reported to occur as primary MMRd (intrinsic or germline Lynch bMMRd) or as secondary MMRd (acquired - not present in the original untreated tumor). The latter occurs after effective treatment and cytoreduction of tumors with TMZ and then selection or induction of mutant MSH6, MSH2, MLH1, or PMS2 proteins and cells which are MMRd and TMZ resistant. The latter is described as an acquired resistance pathway with hotspot mutations in glioma patients (MSH6 p.T1219I).[36] Other resistance pathways are also likely to exist.
References
- ↑ "Temozolomide". Drugs.com. 4 May 2020. Archived from the original on 29 August 2021. Retrieved 7 May 2020.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 "Temodal EPAR". European Medicines Agency (EMA). Archived from the original on 22 October 2020. Retrieved 7 May 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Temodal Capsules - Summary of Product Characteristics (SmPC)". (emc). 24 October 2019. Archived from the original on 20 September 2020. Retrieved 7 May 2020.
- 1 2 "Temozolomide Monograph for Professionals". Drugs.com. Archived from the original on 29 August 2021. Retrieved 25 September 2021.
- 1 2 BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 949. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ↑ "Temozolomide Prices and Temozolomide Coupons - GoodRx". GoodRx. Retrieved 25 September 2021.
- 1 2 "Temodar- temozolomide capsule Temodar- temozolomide injection, powder, lyophilized, for solution". DailyMed. 31 January 2020. Archived from the original on 8 April 2021. Retrieved 7 May 2020.
- 1 2 "Guidance on the use of temozolomide for the treatment of recurrent malignant glioma (brain cancer)" (PDF). 3 March 2016. Archived from the original on 11 July 2021. Retrieved 7 May 2020. Lay summary.
{{cite web}}: Cite uses deprecated parameter|lay-url=(help) - ↑ Farago AF, Yeap BY, Stanzione M, Hung YP, Heist RS, Marcoux JP, et al. (October 2019). "Combination Olaparib and Temozolomide in Relapsed Small-Cell Lung Cancer". Cancer Discov. 9 (10): 1372–1387. doi:10.1158/2159-8290.CD-19-0582. PMC 7319046. PMID 31416802.
- 1 2 3 4 Austria-Codex (in Deutsch). Vienna: Österreichischer Apothekerverlag. 2018. Temodal 5 mg-Hartkapseln.
- ↑ Sitbon Sitruk L, Sanson M, Prades M, Lefebvre G, Schubert B, Poirot C (November 2010). "Chimiothérapie à gonadotoxicité inconnue et préservation de la fertilité: Exemple du témozolomide" [Unknown gonadotoxicity chemotherapy and preservation of fertility: example of Temozolomide]. Gynécologie, Obstétrique & Fertilité (in français). 38 (11): 660–2. doi:10.1016/j.gyobfe.2010.09.002. PMID 21030284.
- ↑ Jacinto FV, Esteller M (August 2007). "MGMT hypermethylation: a prognostic foe, a predictive friend". DNA Repair. 6 (8): 1155–60. doi:10.1016/j.dnarep.2007.03.013. PMID 17482895.
- ↑ Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R (March 2005). "MGMT gene silencing and benefit from temozolomide in glioblastoma" (PDF). The New England Journal of Medicine. 352 (10): 997–1003. doi:10.1056/NEJMoa043331. PMID 15758010. Archived (PDF) from the original on 2018-07-19. Retrieved 2021-08-09.
- ↑ Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO, European Organisation for Research Treatment of Cancer Brain Tumour Radiation Oncology Groups, National Cancer Institute of Canada Clinical Trials Group) (May 2009). "Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial". The Lancet. Oncology. 10 (5): 459–66. doi:10.1016/S1470-2045(09)70025-7. PMID 19269895.
- 1 2 3 Sansom C (July 2009). "Temozolomide – birth of a blockbuster" (PDF). Chemistry World: 48–51. Archived (PDF) from the original on 2020-10-22. Retrieved 2021-08-09.
- ↑ "Temodal: EPAR – Scientific Discussion" (PDF). European Medicines Agency. 2005-12-13. Archived (PDF) from the original on 2021-08-29. Retrieved 2021-08-09.
- ↑ Dinnendahl, V; Fricke, U, eds. (2016). Arzneistoff-Profile (in Deutsch). Vol. 9 (29 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. ISBN 978-3-7741-9846-3.
- ↑ Sperry, Jeffrey B.; Stone, Shane; Azuma, Michael; Barrett, Connor (2021). "Importance of Thermal Stability Data to Avoid Dangerous Reagents: Temozolomide Case Study". Organic Process Research & Development. 25 (7): 1690–1700. doi:10.1021/acs.oprd.1c00206.
- ↑ Derek Lowe (12 July 2021). "Temozolomide Is Explosive". Archived from the original on 9 August 2021. Retrieved 9 August 2021.
- ↑ "Malcolm Steven – interview". Cancer Research UK impact & achievements page. 2013-08-22. Archived from the original on 14 March 2012.
- ↑ Newlands ES, Stevens MF, Wedge SR, Wheelhouse RT, Brock C (January 1997). "Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials". Cancer Treatment Reviews. 23 (1): 35–61. doi:10.1016/S0305-7372(97)90019-0. PMID 9189180.
- ↑ "Drug Approval Package: Temodar (Temozolomide) NDA# 021029". U.S. Food and Drug Administration (FDA). 30 March 2001. Archived from the original on 31 March 2021. Retrieved 7 May 2020.
- ↑ "Drug Approval Package: Temodar NDA #022277". U.S. Food and Drug Administration (FDA). 24 November 2009. Archived from the original on 28 March 2021. Retrieved 7 May 2020.
- ↑ Gilbert MR (March 2006). "New treatments for malignant gliomas: careful evaluation and cautious optimism required". Annals of Internal Medicine. 144 (5): 371–3. doi:10.7326/0003-4819-144-5-200603070-00015. PMID 16520480. S2CID 21181702.
- ↑ Pyrko P, Schönthal AH, Hofman FM, Chen TC, Lee AS (October 2007). "The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas". Cancer Research. 67 (20): 9809–16. doi:10.1158/0008-5472.CAN-07-0625. PMID 17942911. Archived from the original on 2012-07-07. Retrieved 2021-08-09.
- ↑ Sheehan J, Cifarelli CP, Dassoulas K, Olson C, Rainey J, Han S (August 2010). "Trans-sodium crocetinate enhancing survival and glioma response on magnetic resonance imaging to radiation and temozolomide". Journal of Neurosurgery. 113 (2): 234–9. doi:10.3171/2009.11.JNS091314. PMID 20001586.
- ↑ "Safety and Efficacy Study of Trans Sodium Crocetinate (TSC) With Concomitant Radiation Therapy and Temozolomide in Newly Diagnosed Glioblastoma (GBM)". ClinicalTrials.gov. November 2011. Archived from the original on 2014-10-21. Retrieved 2016-02-01.
- ↑ Da Fonseca CO, Teixeira RM, Silva JC, De Saldanha Da Gama Fischer J, Meirelles OC, Landeiro JA, Quirico-Santos T (December 2013). "Long-term outcome in patients with recurrent malignant glioma treated with Perillyl alcohol inhalation". Anticancer Research. 33 (12): 5625–31. PMID 24324108.
- ↑ Cho HY, Wang W, Jhaveri N, Lee DJ, Sharma N, Dubeau L, Schönthal AH, Hofman FM, Chen TC (August 2014). "NEO212, temozolomide conjugated to perillyl alcohol, is a novel drug for effective treatment of a broad range of temozolomide-resistant gliomas". Molecular Cancer Therapeutics. 13 (8): 2004–17. doi:10.1158/1535-7163.mct-13-0964. PMID 24994771.
- ↑ Chen TC, Cho HY, Wang W, Nguyen J, Jhaveri N, Rosenstein-Sisson R, Hofman FM, Schönthal AH (March 2015). "A novel temozolomide analog, NEO212, with enhanced activity against MGMT-positive melanoma in vitro and in vivo". Cancer Letters. 358 (2): 144–51. doi:10.1016/j.canlet.2014.12.021. PMID 25524552.
- ↑ Chen TC, Cho HY, Wang W, Barath M, Sharma N, Hofman FM, Schönthal AH (May 2014). "A novel temozolomide-perillyl alcohol conjugate exhibits superior activity against breast cancer cells in vitro and intracranial triple-negative tumor growth in vivo". Molecular Cancer Therapeutics. 13 (5): 1181–93. doi:10.1158/1535-7163.mct-13-0882. PMID 24623736.
- ↑ Ueno T, Ko SH, Grubbs E, Yoshimoto Y, Augustine C, Abdel-Wahab Z, Cheng TY, Abdel-Wahab OI, Pruitt SK, Friedman HS, Tyler DS (March 2006). "Modulation of chemotherapy resistance in regional therapy: a novel therapeutic approach to advanced extremity melanoma using intra-arterial temozolomide in combination with systemic O6-benzylguanine". Molecular Cancer Therapeutics. 5 (3): 732–8. doi:10.1158/1535-7163.MCT-05-0098. PMID 16546988. Archived from the original on 2021-08-29. Retrieved 2021-08-09.
- ↑ Quinn JA, Jiang SX, Reardon DA, Desjardins A, Vredenburgh JJ, Rich JN, Gururangan S, Friedman AH, Bigner DD, Sampson JH, McLendon RE, Herndon JE, Walker A, Friedman HS (March 2009). "Phase II trial of temozolomide plus o6-benzylguanine in adults with recurrent, temozolomide-resistant malignant glioma". Journal of Clinical Oncology. 27 (8): 1262–7. doi:10.1200/JCO.2008.18.8417. PMC 2667825. PMID 19204199.
- ↑ "Investigative Engineered Bone Marrow Cell Therapy". Fred Hutchinson Cancer Research Center. 2011-05-23. Archived from the original on 2020-11-29. Retrieved 2021-08-09.
- ↑ Dall'oglio S, D'Amico A, Pioli F, Gabbani M, Pasini F, Passarin MG, Talacchi A, Turazzi S, Maluta S (December 2008). "Dose-intensity temozolomide after concurrent chemoradiotherapy in operated high-grade gliomas". Journal of Neuro-Oncology. 90 (3): 315–9. doi:10.1007/s11060-008-9663-9. PMID 18688571. S2CID 21517366.
- ↑ Touat, M; Li, YY; Boynton, AN; Spurr, LF; Iorgulescu, JB; Bohrson, CL; Cortes-Ciriano, I; Birzu, C; Geduldig, JE; Pelton, K; Lim-Fat, MJ; Pal, S; Ferrer-Luna, R; Ramkissoon, SH; Dubois, F; Bellamy, C; Currimjee, N; Bonardi, J; Qian, K; Ho, P; Malinowski, S; Taquet, L; Jones, RE; Shetty, A; Chow, KH; Sharaf, R; Pavlick, D; Albacker, LA; Younan, N; Baldini, C; Verreault, M; Giry, M; Guillerm, E; Ammari, S; Beuvon, F; Mokhtari, K; Alentorn, A; Dehais, C; Houillier, C; Laigle-Donadey, F; Psimaras, D; Lee, EQ; Nayak, L; McFaline-Figueroa, JR; Carpentier, A; Cornu, P; Capelle, L; Mathon, B; Barnholtz-Sloan, JS; Chakravarti, A; Bi, WL; Chiocca, EA; Fehnel, KP; Alexandrescu, S; Chi, SN; Haas-Kogan, D; Batchelor, TT; Frampton, GM; Alexander, BM; Huang, RY; Ligon, AH; Coulet, F; Delattre, JY; Hoang-Xuan, K; Meredith, DM; Santagata, S; Duval, A; Sanson, M; Cherniack, AD; Wen, PY; Reardon, DA; Marabelle, A; Park, PJ; Idbaih, A; Beroukhim, R; Bandopadhayay, P; Bielle, F; Ligon, KL (April 2020). "Mechanisms and therapeutic implications of hypermutation in gliomas". Nature. 580 (7804): 517–523. Bibcode:2020Natur.580..517T. doi:10.1038/s41586-020-2209-9. PMC 8235024. PMID 32322066.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- "Temozolomide (Temodal)". Cancer Research UK. Archived from the original on 2021-08-29. Retrieved 2021-08-09.
- Kaloshi G, Benouaich-Amiel A, Diakite F, et al. (May 2007). "Temozolomide for low-grade gliomas: predictive impact of 1p/19q loss on response and outcome". Neurology. 68 (21): 1831–6. doi:10.1212/01.wnl.0000262034.26310.a2. PMID 17515545. Lay summary.
{{cite journal}}: Cite uses deprecated parameter|lay-url=(help)