Prednisolamate
 | |
| Clinical data | |
|---|---|
| Trade names | Etaproctene (with lidocaine and tetryzoline) |
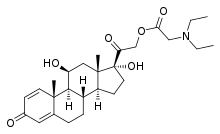
| Other names | Prednisolone 21-diethylaminoacetate; 11β,17α,21-Trihydroxypregna-1,4-diene-3,20-dione 21-N,N-diethylglycine ester |
| Drug class | Corticosteroid; Glucocorticoid |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.024.605 |
| Chemical and physical data | |
| Formula | C27H39NO6 |
| Molar mass | 473.610 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Prednisolamate, also known as prednisolone 21-diethylaminoacetate, is a synthetic corticosteroid.[1][2] It is or was a component of Etaproctene, which contains lidocaine, prednisolamate hydrochloride, and tetryzoline.[3]
References
- ↑ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 1012–. ISBN 978-1-4757-2085-3.
- ↑ Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 871–. ISBN 978-3-88763-075-1.
- ↑ Martindale W, et al. (Royal Pharmaceutical Society of Great Britain. Dept. of Pharmaceutical Sciences) (1993). The Extra Pharmacopoeia. Pharmaceutical Press. p. 737. ISBN 978-0-85369-300-0.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.