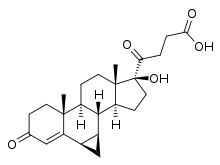
Prorenoic acid
 | |
| Clinical data | |
|---|---|
| Other names | Prorenoate; 6α,7α-Dihydro-17-hydroxy-3-oxo-3'H-cyclopropa(6,7)-17α-pregna-4,6-diene-21-carboxylic acid |
| Drug class | Antimineralocorticoid |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| Chemical and physical data | |
| Formula | C23H31O4 |
| Molar mass | 371.497 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Prorenoic acid, or prorenoate, is a synthetic steroidal antimineralocorticoid which was never marketed.[1][2][3][4]
See also
References
- ↑ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 1036–. ISBN 978-1-4757-2085-3.
- ↑ Claire M, Rafestin-Oblin ME, Michaud A, Roth-Meyer C, Corvol P (1979). "Mechanism of action of a new antialdosterone compound, prorenone". Endocrinology. 104 (4): 1194–200. doi:10.1210/endo-104-4-1194. PMID 436757.
- ↑ Netchitailo P, Delarue C, Perroteau I, Jegou S, Tonon MC, Leroux P, Leboulenger F, Kusmierek MC, Capron MH, Vaudry H (1982). "Effect of aldosterone antagonists on mineralocorticoid synthesis in vitro. Inhibition of aldosterone production by prorenoate-K". Eur. J. Pharmacol. 77 (4): 243–9. doi:10.1016/0014-2999(82)90125-x. PMID 6277668.
- ↑ I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 234–. ISBN 978-94-011-4439-1.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.