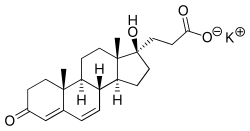
Potassium canrenoate
 | |
| Clinical data | |
|---|---|
| Other names | SC-14266 |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Intravenous |
| ATC code | |
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Excretion | Renal and fecal |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.016.868 |
| Chemical and physical data | |
| Formula | C22H29KO4 |
| Molar mass | 396.568 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Potassium canrenoate (INN, JAN) or canrenoate potassium (USAN) (brand names Venactone, Soldactone), also known as aldadiene kalium,[1] the potassium salt of canrenoic acid, is an aldosterone antagonist of the spirolactone group.[2] Like spironolactone, it is a prodrug, and is metabolized to active canrenone in the body.[3][4]
Potassium canrenoate is notable in that it is the only clinically used antimineralocorticoid which is available for parenteral administration (specifically intravenous)[4][5] as opposed to oral administration.[6]
In the UK it is unlicensed and only used for short term diuresis in oedema or heart failure in neonates or children under specialist initiation and monitoring.
See also
References
- ↑ Hans Selye (17 April 2013). Hormones and Resistance: Part 1 and. Springer Science & Business Media. pp. 186–. ISBN 978-3-642-65192-2.
- ↑ R.A. Hill; H.L.J. Makin; D.N. Kirk; G.M. Murphy (23 May 1991). Dictionary of Steroids. CRC Press. pp. 656–. ISBN 978-0-412-27060-4.
- ↑ Alfred Burger; Manfred E. Wolff (1996). Burger's Medicinal Chemistry and Drug Discovery: Therapeutic agents. Wiley. ISBN 978-0-471-57557-3.
- 1 2 Carl Waldmann; Neil Soni; Andrew Rhodes (27 November 2008). Oxford Desk Reference: Critical Care. OUP Oxford. pp. 187–. ISBN 978-0-19-922958-1.
- ↑ H. Jaap Bonjer (21 June 2017). Surgical Principles of Minimally Invasive Procedures: Manual of the European Association of Endoscopic Surgery (EAES). Springer. pp. 136–. ISBN 978-3-319-43196-3.
- ↑ Kolkhof P, Bärfacker L (2017). "30 YEARS OF THE MINERALOCORTICOID RECEPTOR: Mineralocorticoid receptor antagonists: 60 years of research and development". J. Endocrinol. 234 (1): T125–T140. doi:10.1530/JOE-16-0600. PMC 5488394. PMID 28634268.
| Sulfonamides (and etacrynic acid) |
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Potassium-sparing (at CD) |
| ||||||||
| Osmotic diuretics (PT, DL) | |||||||||
| Vasopressin receptor inhibitors (DCT and CD) |
| ||||||||
| Other |
| ||||||||
| Combination products | |||||||||
| |||||||||
Mineralocorticoids and antimineralocorticoids (H02) | |
|---|---|
| Mineralocorticoids | |
| Antimineralocorticoids | |
| Synthesis modifiers | |
| |
Mineralocorticoid receptor modulators | |||||
|---|---|---|---|---|---|
| MR |
| ||||
| |||||
Progesterone receptor modulators | |||||||
|---|---|---|---|---|---|---|---|
| PR |
| ||||||
| mPR (PAQR) |
| ||||||
| |||||||
Androgen receptor modulators | |||||||
|---|---|---|---|---|---|---|---|
| AR |
| ||||||
| GPRC6A |
| ||||||
| |||||||
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.