Methylepitiostanol
 | |
| Clinical data | |
|---|---|
| Other names | Epistane; Hemapolin; Havoc; Epi Plex; Methylepithiostanol; Methepitiostane; 17α-Methylepitiostanol; 2α,3α-Epithio-17α-methyl-4,5α-dihydrotestosterone; 2α,3α-Epithio-17α-methyl-DHT |
| Routes of administration | By mouth[1] |
| Drug class | Androgen; Anabolic steroid; Antiestrogen |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C20H32OS |
| Molar mass | 320.54 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
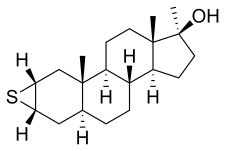
Methylepitiostanol, known by the nicknames Epistane, Hemapolin, Havoc, and Epi Plex, is a synthetic and orally active anabolic–androgenic steroid (AAS) of the dihydrotestosterone (DHT) group which was first described in the literature in 1974 but was never marketed for medical use.[1][2][3] It is the 17α-methylated derivative of epitiostanol, an AAS and antiestrogen which was formerly used in the treatment of breast cancer in Japan.[1][2] Similarly to mepitiostane, methylepitiostanol is an orally active variant of epitiostanol.[1][2] Due to its C17α methyl group, the drug is considered to have a high potential for hepatotoxicity.[1]
Methylepitiostanol surfaced on the internet as a novel designer steroid in dietary supplements around 2009.[1] It was identified in 2015 in over 30 products sold online that listed it as an ingredient on their product label.[1]
It became a controlled substance in 2014 with the passage of the Designer Anabolic Steroid Control Act, being one of the 27 new steroids explicitly listed as controlled by the Act.
Chemistry
Methylepitiostanol, also known as 2α,3α-epithio-17α-methyl-4,5α-dihydrotestosterone (2α,3α-epithio-17α-methyl-DHT) or as 2α,3α-epithio-17α-methyl-5α-androstan-17β-ol, is a synthetic androstane steroid and a 17α-alkylated derivative of DHT.[1][2] It is closely related to epitiostanol (2α,3α-epithio-DHT) and mepitiostane (epitiostanol 17-methyloxycyclopentyl ether).[1]
References
- 1 2 3 4 5 6 7 8 9 10 Rahnema, C. D.; Crosnoe, L. E.; Kim, E. D. (March 2015). "Designer steroids – over-the-counter supplements and their androgenic component: review of an increasing problem". Andrology. 3 (2): 150–155. doi:10.1111/andr.307. ISSN 2047-2927. PMID 25684733. S2CID 6999218.
- 1 2 3 4 Miyake, Tamotsu; Uchida, Kiyohisa; Kakushi, Hisato; Nomura, Yasuharu; Kadowaki, Masumi; Miyata, Kenji; Hanafusa, Tomoyuki; Muranaka, Ri-ichi (1974). "2α, 3α-EPITHIO-5α-ANDROSTAN-17β-YL 1-METHOXY CYCLOPENTYL ETHER (10364-S), A NEW ORALLY ACTIVE ANABOLIC-ANDROGENIC STEROID". The Japanese Journal of Pharmacology. 24 (4): 551–558. doi:10.1254/jjp.24.551. ISSN 0021-5198. PMID 4455965.
- ↑ "2,3-Thioepoxy madol".
- ↑ Okano, Masato; Sato, Mitsuhiko; Ikekita, Ayako; Kageyama, Shinji (November–December 2009). "Analysis of non-ketoic steroids 17α-methylepithiostanol and desoxymethyl-testosterone in dietary supplements". Drug Testing and Analysis. 1 (11–12): 518–525. doi:10.1002/dta.72. ISSN 1942-7611. PMID 20355167.
- ↑ Okano M, Sato M, Ikekita A, Kageyama S (2009). "Analysis of non-ketoic steroids 17alpha-methylepithiostanol and desoxymethyl- testosterone in dietary supplements". Drug Test Anal. 1 (11–12): 518–25. doi:10.1002/dta.72. PMID 20355167.