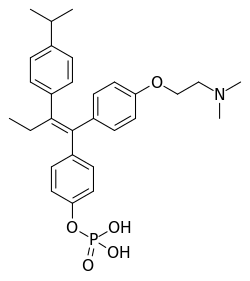
Miproxifene phosphate
 | |
| Clinical data | |
|---|---|
| Other names | TAT-59; Iproxifene |
| Routes of administration | Oral |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C29H36NO5P |
| Molar mass | 509.583 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Miproxifene phosphate (former developmental code name TAT-59) is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group[1] that was under development in Japan for the treatment of breast cancer but was abandoned and never marketed.[2][3][4][5] It reached phase III clinical trials for this indication before development was discontinued.[2][5] The drug is a phosphate ester and prodrug of miproxifene (DP-TAT-59) with improved water solubility that was better suited for clinical development.[2][3][6][7] Miproxifene has been found to be 3- to 10-fold as potent as tamoxifen in inhibiting breast cancer cell growth in in vitro models.[2][5][4] It is a derivative of afimoxifene (4-hydroxytamoxifen) in which an additional 4-isopropyl group is present in the β-phenyl ring.[8]
References
- ↑ Miller WR, Ingle JN (8 March 2002). Endocrine Therapy in Breast Cancer. CRC Press. pp. 53–. ISBN 978-0-203-90983-6.
- 1 2 3 4 "Miproxifene". AdisInsight. Springer Nature Switzerland AG.
- 1 2 Stella V, Borchardt R, Hageman M, Oliyai R, Maag H, Tilley J (12 March 2007). Prodrugs: Challenges and Rewards. Springer Science & Business Media. pp. 168–169. ISBN 978-0-387-49782-2.
- 1 2 Kelloff GJ, Hawk ET, Sigman CC (17 August 2008). Cancer Chemoprevention: Volume 2: Strategies for Cancer Chemoprevention. Springer. pp. 251–. ISBN 978-1-59259-768-0.
- 1 2 3 Ottow E, Weinmann H (8 September 2008). Nuclear Receptors as Drug Targets. John Wiley & Sons. pp. 90–. ISBN 978-3-527-62330-3.
- ↑ Stromgaard K, Krogsgaard-Larsen P, Madsen U (19 August 2016). Textbook of Drug Design and Discovery, Fifth Edition. CRC Press. pp. 162–. ISBN 978-1-4987-0279-9.
- ↑ Yang HC, Yeh WK, McCarthy JR (22 November 2013). Enzyme Technologies: Pluripotent Players in Discovering Therapeutic Agent. Wiley. pp. 166–. ISBN 978-1-118-73989-1.
- ↑ Oettel M, Schillinger E (6 December 2012). Estrogens and Antiestrogens I: Physiology and Mechanisms of Action of Estrogens and Antiestrogens. Springer Science & Business Media. pp. 58–60. ISBN 978-3-642-58616-3.
External links
- "Miproxifene". AdisInsight. Springer Nature Switzerland AG.