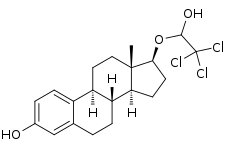
Cloxestradiol
 | |
| Clinical data | |
|---|---|
| Other names | 17-(2,2,2-Trichloroethoxy)estradiol; 17-(1-Hydroxy-2,2,2-trichloroethoxy)estra-1,3,5(10-trien-3-ol |
| Drug class | Estrogen; Estrogen ether |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C20H25Cl3O3 |
| Molar mass | 419.77 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Cloxestradiol (INN), also known as 17-(2,2,2-trichloroethoxy)estradiol, is a synthetic, steroidal estrogen which was never marketed.[1] It is an analogue of estradiol with a 2,2,2-trichloroethoxy substitution.[1] The O,O-diacetate derivative, cloxestradiol acetate (brand name Genovul), has been marketed as an estrogen.[1]
See also
References
- 1 2 3 Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 308–. ISBN 978-1-4757-2085-3.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.